超快速、远程控制的质子化反应可以改变光敏色素的结构
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
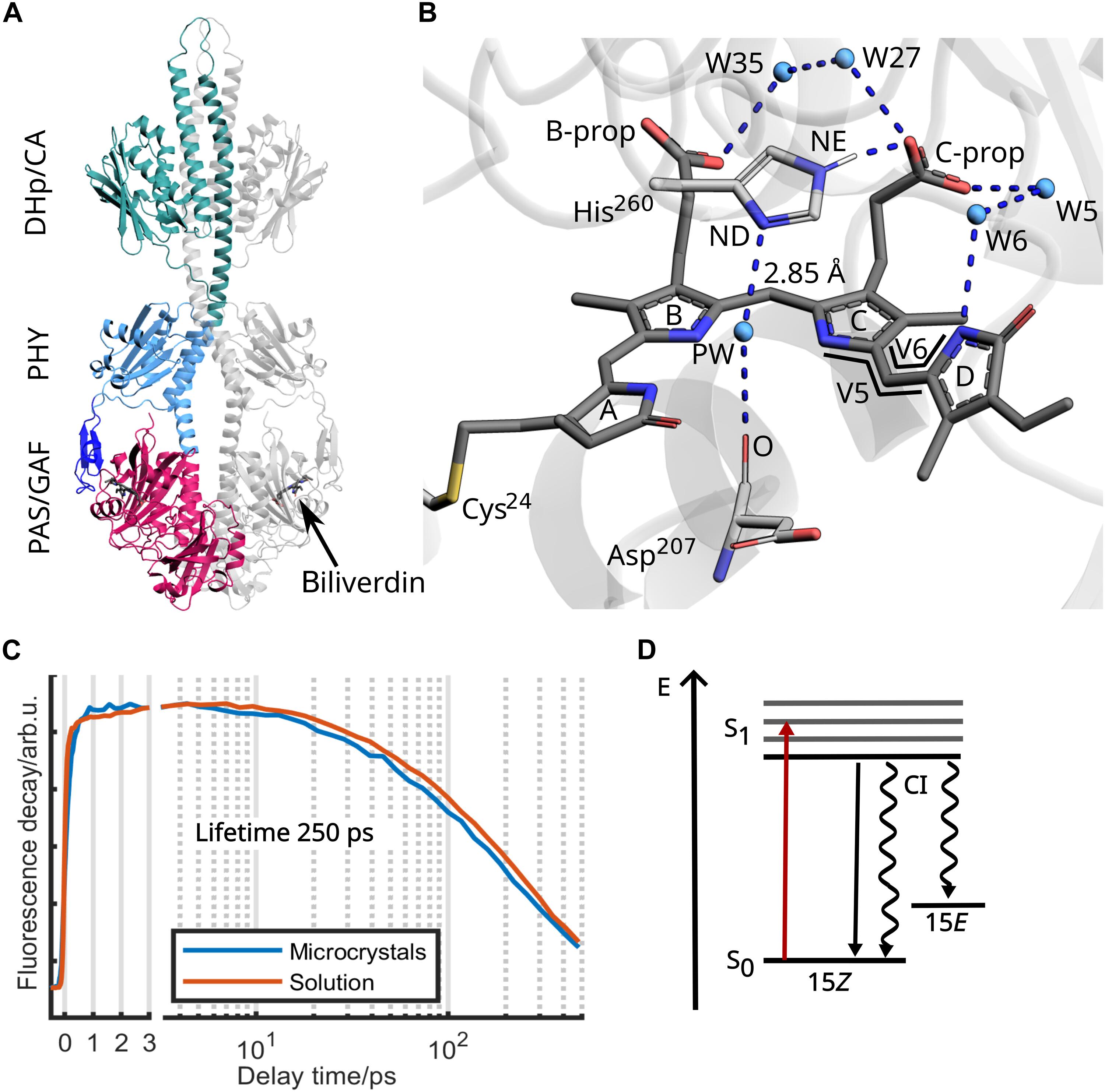
在光活性蛋白中,发色团和蛋白基质之间的偶联是精细调节的。质子转移反应可以介导这种耦合,如质子耦合电子转移和激发态质子转移。涉及质子位错的其他机制可能存在,但仍未被发现。在这里,我们展示了来自耐辐射球菌的光敏色素的飞秒晶体电影。这些结构揭示了d环在激发态下旋转的空间守恒机制。我们观察到一个保守的氢键网络在300秒内重排,这先于发色团的异构化反应。借助分子模型和飞秒红外光谱独立证实,我们将这些变化归因于严格保守的组氨酸-260的质子化移位。虽然这个组氨酸靠近发色团的光激发π轨道,但它不是它们的直接组成部分。我们提出这种“遥控”质子转移将光激发几乎瞬间传递到蛋白质基质。该机制可广泛用于将辅因子信号转导到其宿主酶。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ultrafast, remote-controlled protonation reaction enables structural changes in a phytochrome
In photoactive proteins, coupling between the chromophore and protein matrix is exquisitely tuned. Proton transfer reactions can mediate this coupling, as in proton-coupled electron transfer and excited-state proton transfer. Additional mechanisms involving proton dislocations may exist but remain undiscovered. Here, we present a femtosecond crystallographic movie of the phytochrome from Deinococcus radiodurans. The structures reveal a space-conserving mechanism for rotation of the D-ring in the excited state. We observe rearrangement of a conserved hydrogen bond network within 300 fs, which precedes the isomerization reaction of the chromophore. Aided by molecular modeling and independently confirmed by femtosecond infrared spectroscopy, we attribute these changes to a protonation shift of the strictly conserved histidine-260. Although this histidine lies close to the photoexcited π-orbitals of the chromophore, it is not directly part of them. We propose that this “remote-controlled” proton transfer relays photoexcitation near-instantaneously to the protein matrix. This mechanism may be widely used to transduce cofactor signals to their hosting enzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


