热电凝胶中反应动力学和热力学的揭示:通往高效热能收集的途径
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
热电(TG)系统通过利用在温度梯度上建立的与温度相关的电化学电位来发电。虽然以前的研究将性能的提高归因于溶剂化结构变化导致的反应熵的增加,但熵变化与反应动力学之间的相互作用尚未通过电化学分析进行系统的探讨。本研究介绍了甲酰胺(FA)/水基TG水凝胶的综合研究。电化学分析表明,与纯水系统相比,FA/水系统表现出明显更高的反应熵,从而导致热功率增强。更重要的是,首次使用固定电极对TG水凝胶的反应动力学进行了研究。结果表明,低FA浓度促进了氧化还原离子的脱溶,从而加快了反应动力学,增加了短路电流密度。此外,FA作为水的稳定剂,从而降低了氧化还原反应的活化能,使总热电功率提高了60%。这项工作提供了对FA/水TG水凝胶的基本机制的见解,并强调了反应动力学在优化热能收集应用的热电性能方面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
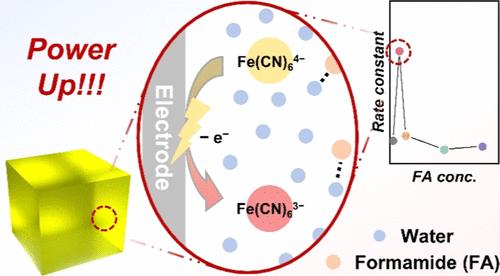
Unraveling Reaction Kinetics and Thermodynamics in Thermogalvanic Hydrogels: A Pathway to Efficient Thermal Energy Harvesting
Thermogalvanic (TG) systems generate electricity by exploiting the temperature-dependent electrochemical potential established across a temperature gradient. While previous studies have attributed performance enhancements to increased reaction entropy driven by changes in solvation structure, the interplay between entropy variations and reaction kinetics has not been systematically explored through electrochemical analysis. This study presents a comprehensive investigation of formamide (FA)/water-based TG hydrogels. Electrochemical analyses reveal that FA/water systems exhibit significantly higher reaction entropy compared to pure water systems, thus leading to enhanced thermopower. More importantly, the reaction kinetics of the TG hydrogels are examined using a stationary electrode for the first time. The results indicate that low FA concentrations promote the desolvation of redox ions, thereby accelerating the reaction kinetics and increasing the short-circuit current density. In addition, FA acts as a stabilizer for water, thereby lowering the activation energy of the redox reaction and resulting in a remarkable 60% improvement in overall thermoelectric power. This work provides insights into the fundamental mechanisms governing FA/water TG hydrogels and emphasizes the critical role of reaction kinetics in optimizing the thermoelectric performance for thermal energy harvesting applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


