纳米粒子阳离子交换过程中连续界面反应形成固溶体
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由两个或多个端元之间形成的固溶体组成的纳米颗粒通常表现出可调和协同特性。固溶纳米颗粒的合成通常需要同时递送多种试剂,这些试剂的反应性经过仔细平衡,以便所有组成元素同时均匀结合。阳离子交换反应为将固溶体引入纳米颗粒提供了一种替代的合成策略;这些固溶形成反应也被认为需要同时输送试剂。在这里,我们表明,在纳米颗粒阳离子交换过程中,固溶体的形成可以从一系列原位连续反应中出现,这些反应在试剂同时传递的整个反应中以逐步的方式发生。研究表明,在Cu1.8S纳米棒与Ni2+和Co2+同时共交换的过程中,需要在铁长石Cu1.8S与硫化镍、硫化钴或镍钴硫化之间形成一个事先存在的界面,从而形成镍钴硫化固溶体NixCo9-xS8。我们的数据表明,在Ni2+和Co2+同时共交换的过程中,Ni2+结合到一个瞬态亚稳的钴硫化物相中,稳定为NixCo9-xS8。然后,我们利用这些见解选择性地合成具有组成梯度的NixCo9-xS8纳米棒和具有均匀组成的NixCo9-xS8纳米棒。因此,界面反应性和结构稳定性可以影响同时共交换过程中的阳离子迁移,并最终促进固溶体的形成,从而将复杂的特征(包括成分梯度)引入胶体纳米颗粒中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
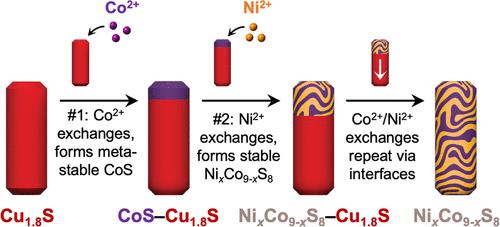
Solid Solution Formation from Sequential Interfacial Reactions during Nanoparticle Cation Exchange
Nanoparticles that consist of solid solutions formed between two or more end members often exhibit tunable and synergistic properties. The synthesis of solid-solution nanoparticles typically requires simultaneous delivery of multiple reagents with carefully balanced reactivities so that all constituent elements combine uniformly at the same time. Cation exchange reactions provide an alternative postsynthetic strategy for introducing solid solutions into nanoparticles; these solid-solution formation reactions also are assumed to require simultaneous delivery of reagents. Here, we show that solid solution formation during nanoparticle cation exchange can emerge from a series of in situ sequential reactions that occur in a stepwise manner throughout a reaction where reagents are delivered simultaneously. We demonstrate that during simultaneous coexchange of Cu1.8S nanorods with Ni2+ and Co2+, a pre-existing interface between roxbyite Cu1.8S and either nickel sulfide, cobalt sulfide, or a nickel–cobalt sulfide is required to form the nickel–cobalt sulfide solid solution NixCo9–xS8. Our data suggest that during simultaneous coexchange of Ni2+ and Co2+, Ni2+ incorporates into a transient and metastable cobalt sulfide phase to stabilize it as NixCo9–xS8. We then leverage these insights to selectively synthesize NixCo9–xS8 nanorods having a composition gradient and NixCo9–xS8 nanorods having uniform compositions throughout. Interfacial reactivity and structural stability can therefore influence cation migration during simultaneous coexchange and ultimately facilitate solid solution formation, leading to the introduction of complex features, including composition gradients, into colloidal nanoparticles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


