染色体外环状DNA促进炎症和肝细胞癌的发展
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
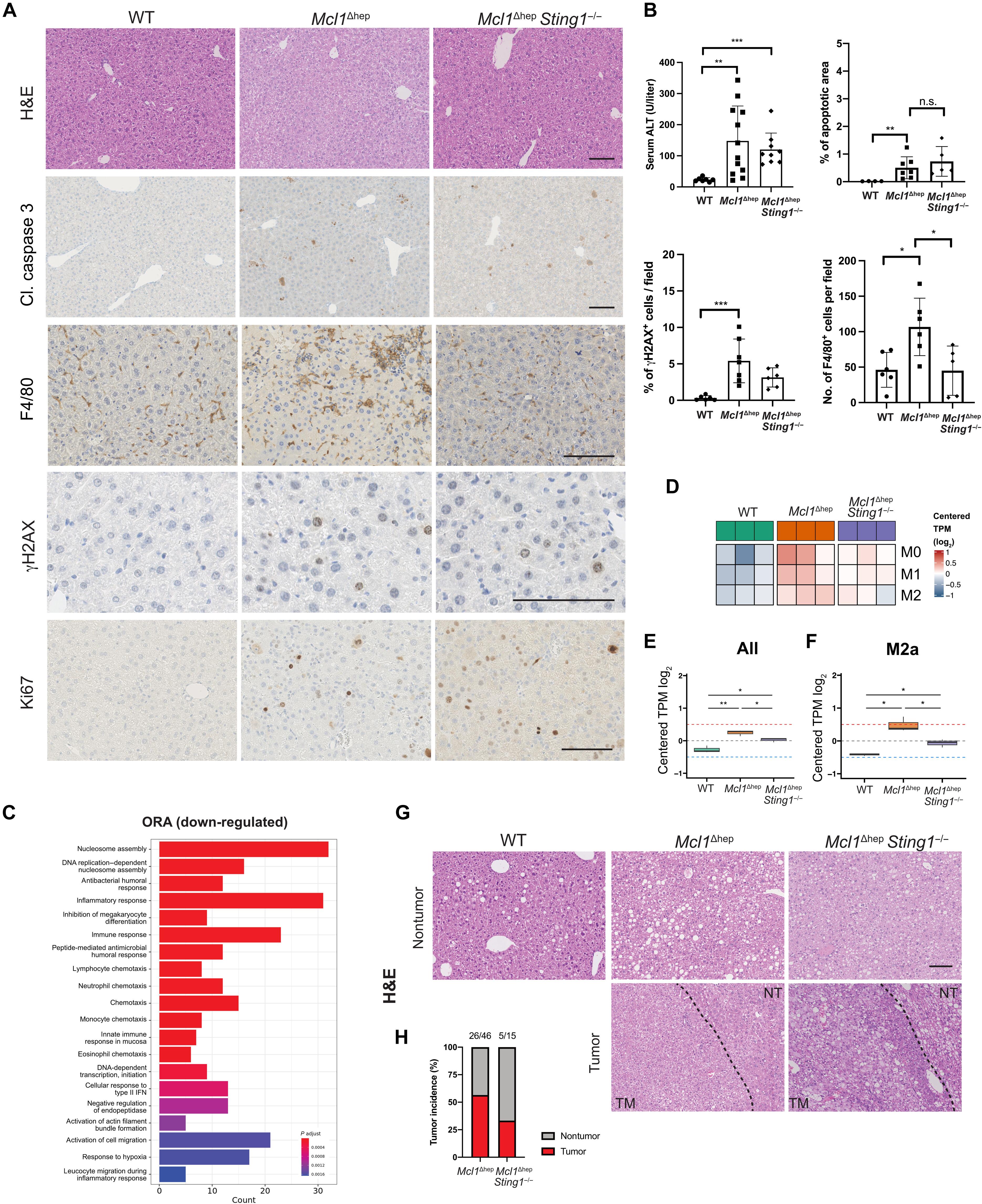
在人类慢性肝病(CLD)和肝细胞癌(HCC)中微核增加的最初报道20年后,它们在HCC发展中的作用仍然知之甚少。在这里,我们发现肝细胞中的微核通过增加染色体外环状DNA (eccDNA)水平触发肝脏免疫反应并促进HCC的发展。CLD模型(Mcl1 Δhep小鼠)的肝脏显示微核和eccDNA水平升高。环状测序证实微核中的eccDNA水平高于初代核。我们开发的核分离DNA纤维(NuSeF)分析表明,微核更容易受到复制胁迫,表现出增加的复制叉减慢。比较不同的小鼠肝脏疾病模型表明,高eccDNA与肿瘤发病率增加相关。eccDNA是一种强免疫刺激剂,通过cGAS-STING通路促进肝细胞和免疫细胞之间的串扰。在Mcl1 Δhep小鼠中删除Sting1可降低免疫细胞趋化性和肿瘤发生率。我们的研究结果表明,来自微核的eccDNA介导了CLD中炎症驱动的肝癌发生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Extrachromosomal circular DNA promotes inflammation and hepatocellular carcinoma development
Two decades after the initial report on increased micronuclei in human chronic liver disease (CLD) and hepatocellular carcinoma (HCC), their role in HCC development is still poorly understood. Here, we show that micronuclei in hepatocytes trigger a hepatic immune response and promote HCC development via an increased level of extrachromosomal circular DNA (eccDNA). Livers of a CLD model (Mcl1Δhep mice) show increased micronuclei and eccDNA levels. Circular sequencing confirms higher eccDNA levels in micronuclei compared to primary nuclei. The nuclei-segregated DNA fiber (NuSeF) assay we developed demonstrates that micronuclei are more susceptible to replication stress, exhibiting increased replication fork slowing. Comparing different murine liver disease models reveals that high eccDNA correlates with an increased tumor incidence. eccDNA is a strong immunostimulant and promotes a cross-talk between hepatocytes and immune cells through the cGAS-STING pathway. Deletion of Sting1 in Mcl1Δhep mice reduces immune cell chemotaxis and tumor incidence. Our findings suggest that eccDNA from micronuclei mediates inflammation-driven liver carcinogenesis in CLD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


