在慢性抗原暴露下,nr4a3缺陷的CAR - T细胞中,FOS表达增强可提高肿瘤清除率并抵抗衰竭
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
嵌合抗原受体(CAR) T细胞在肿瘤微环境中的功能障碍是影响其治疗实体瘤疗效的主要障碍。通过对胶质瘤患者肿瘤浸润T细胞的单细胞RNA测序分析,发现NR4A家族基因与T细胞衰竭密切相关,并与功能失调基因HAVCR2和TIGIT共表达。值得注意的是,NR4A3敲低的CAR - T细胞对肿瘤表现出增强的细胞毒活性,从而提高肿瘤清除率,延长体内生存期。然而,随着肿瘤负荷的延长,促进的抗疲劳表型减少。这种T细胞功能的下降与NR4A3敲低后慢性抗原暴露诱导的FOS代偿性下调有关。过表达FOS和NR4A3敲低显著增强了CAR - T细胞的抗肿瘤反应,使它们的表型和转录谱偏离衰竭和增加效应功能。这些发现为CAR - T细胞治疗的临床修饰提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
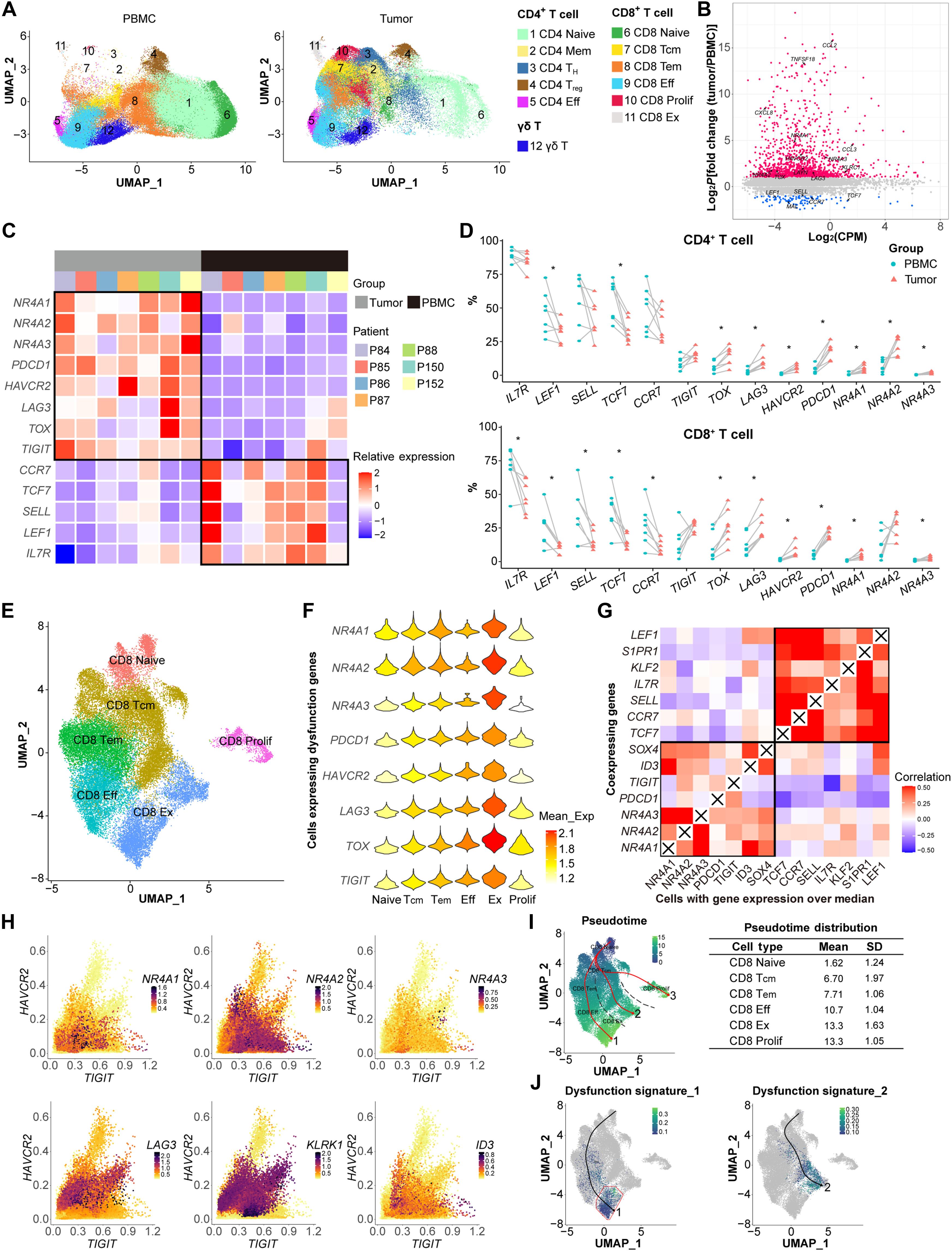
Enhanced FOS expression improves tumor clearance and resists exhaustion in NR4A3-deficient CAR T cells under chronic antigen exposure
The dysfunction of chimeric antigen receptor (CAR) T cells in the tumor microenvironment is a major obstacle to their therapeutic efficacy against solid tumors. Through single-cell RNA sequencing analysis of tumor-infiltrating T cells from patients with glioma, NR4A family genes were identified as closely associated with T cell exhaustion and were coexpressed with dysfunctional genes HAVCR2 and TIGIT. Notably, CAR T cells with NR4A3 knockdown exhibited enhanced cytotoxic activity against tumors, leading to improved tumor clearance and prolonged survival in vivo. However, the promoted antiexhausted phenotype diminished with prolonged tumor burden. This decline in T cell function correlates with the compensatory down-regulation of FOS induced by chronic antigen exposure following NR4A3 knockdown. Overexpressing FOS alongside NR4A3 knockdown robustly boosted the antitumor responses of CAR T cells by skewing their phenotypes and transcriptional profiles away from exhaustion and toward increased effector function. These findings offer a promising strategy for the clinical modification of CAR T cell therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


