表征溶质在细胞层之间的运输:从简化的三室模型中进行伪影校正和参数提取。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
在早期药物开发中,定量测定在transwell插入物上生长的上皮细胞层间溶质转运是评估药物吸收和生物利用度等药代动力学特性的常用方法。为了提高通量和减少可变性,这些分析越来越自动化,包括使用机器人或微流体系统进行时间分辨采样。然而,自动和手动取样都可能引入系统性的伪影,如残余体积保留和表面吸附,从而扭曲浓度时间序列并影响下游分析。为了充分实现自动化测量的潜在精度,我们提出了一个数学校正来考虑采样伪影;然后,将校正后的数据拟合到捕获膜扩散、细胞隔离和代谢损失的三室模型中。该方法在transwell上皮屏障运输试验的数据集上得到了验证。我们认为,考虑的三室模型产生的力学参数比传统的表观渗透率(Papp)测量更有意义。因此,所提出的方法能够更准确地表征分析物与屏障细胞层的相互作用,支持更好地评估化合物在体外运输系统中的行为。在早期药物开发中,定量测定在transwell插入物上生长的上皮细胞层间溶质转运是评估药物吸收和生物利用度等药代动力学特性的常用方法。为了提高通量和减少可变性,这些分析越来越自动化,包括使用机器人或微流体系统进行时间分辨采样。然而,自动和手动取样都可能引入系统性的伪影,如残余体积保留和表面吸附,从而扭曲浓度时间序列并影响下游分析。为了充分实现自动化测量的潜在精度,我们提出了一个数学校正来考虑采样伪影;然后,将校正后的数据拟合到捕获膜扩散、细胞隔离和代谢损失的三室模型中。该方法在transwell上皮屏障运输试验的数据集上得到了验证。我们认为,考虑的三室模型产生的力学参数比传统的表观渗透率(Papp)测量更有意义。因此,所提出的方法能够更准确地表征分析物与屏障细胞层的相互作用,支持更好地评估化合物在体外运输系统中的行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
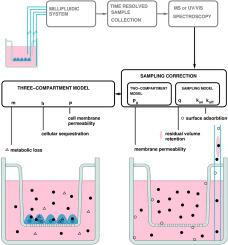
Characterizing solute transport across cell layers: Artifact correction and parameter extraction from a simplified three-compartment model
Quantifying solute transport across epithelial cell layers grown on transwell inserts is a common approach in early-stage drug development to estimate pharmacokinetic properties such as absorption and bioavailability. To increase throughput and reduce variability, these assays are increasingly automated, including the use of robotic or microfluidic systems for time-resolved sampling. However, both automated and manual sampling can introduce systematic artifacts, such as residual volume retention and surface adsorption, that distort concentration time series and affect downstream analysis. To fully realize the potential precision of automated measurements, we propose a mathematical correction to account for sampling artifacts; then, to fit the corrected data to a three-compartment model that captures membrane diffusion, cellular sequestration, and metabolic loss. The method is demonstrated on datasets from transwell epithelial barrier transport assays. We suggest that the considered three-compartment model yields mechanistically more meaningful parameters than the conventional apparent permeability (Papp) measure. The proposed approach thus enables more accurate characterization of analyte interactions with the barrier cell layer, supporting better-informed assessments of compound behavior in vitro transport systems. Quantifying solute transport across epithelial cell layers grown on transwell inserts is a common approach in early-stage drug development to estimate pharmacokinetic properties such as absorption and bioavailability. To increase throughput and reduce variability, these assays are increasingly automated, including the use of robotic or microfluidic systems for time-resolved sampling. However, both automated and manual sampling can introduce systematic artifacts, such as residual volume retention and surface adsorption, that distort concentration time series and affect downstream analysis. To fully realize the potential precision of automated measurements, we propose a mathematical correction to account for sampling artifacts; then, to fit the corrected data to a three-compartment model that captures membrane diffusion, cellular sequestration, and metabolic loss. The method is demonstrated on datasets from transwell epithelial barrier transport assays. We suggest that the considered three-compartment model yields mechanistically more meaningful parameters than the conventional apparent permeability (Papp) measure. The proposed approach thus enables more accurate characterization of analyte interactions with the barrier cell layer, supporting better-informed assessments of compound behavior in vitro transport systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


