Donor-Acceptor-Substituted 5-Azaazulenes
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了稳定的叠氮烯衍生物与二甲基乙炔二羧酸酯反应后,通过扩环反应合成了七元环上既有供体取代基又有受体取代基的5-叠氮烯。得到了区域异构体产物并进行了表征。计算研究了产物的转化机理和激发态能级,表明这些结构可以作为有机光子学应用中发色团设计的切入点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
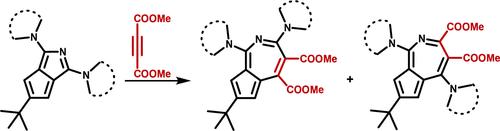
Donor–Acceptor-Substituted 5-Azaazulenes
The synthesis of 5-azaazulenes with both donor and acceptor substituents on their seven-membered rings is reported through the ring expansion of stable azapentalene derivatives upon reaction with dimethyl acetylenedicarboxylate. Regioisomeric products were obtained and characterized. The mechanism of the transformation and the excited state energy levels of the products were studied computationally, suggesting that these structures can be entry points to chromophore design for organic photonics applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


