CRATER肿瘤龛促进CD8+ T细胞参与,并与免疫治疗的成功相对应
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
T细胞介导的肿瘤杀伤是免疫治疗成功的基础。在这里,我们使用斑马鱼内源性黑色素瘤的长期体内成像和高分辨率空间转录组学,以及人类黑色素瘤的多重成像,来识别免疫治疗过程中促进免疫反应的结构域。我们在斑马鱼和人类黑色素瘤的基质-黑素细胞边界处发现了抗原呈递和T细胞接合和保留(CRATERs)的肿瘤区域。陨石坑富含抗原识别分子,是肿瘤中CD8+ T细胞密度最高的地方。在斑马鱼中,CD8+ T细胞与crater内的黑色素瘤细胞形成了长时间的相互作用,这是抗原识别的特征。免疫刺激治疗后,CRATERs扩大,成为活化CD8+ T细胞聚集和杀伤肿瘤的主要位点。在人类中,免疫检查点阻断(ICB)治疗后活检中火山口密度升高与治疗的临床反应相关。肿瘤坑是肿瘤杀伤活性的结构,可作为免疫治疗成功的诊断指标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
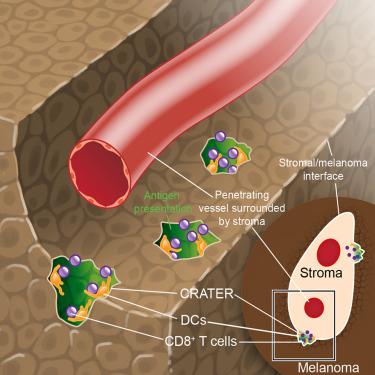
CRATER tumor niches facilitate CD8+ T cell engagement and correspond with immunotherapy success
T cell-mediated tumor killing underlies immunotherapy success. Here, we used long-term in vivo imaging and high-resolution spatial transcriptomics of zebrafish endogenous melanoma, as well as multiplex imaging of human melanoma, to identify domains facilitating the immune response during immunotherapy. We identified cancer regions of antigen presentation and T cell engagement and retention (CRATERs) as pockets at the stroma-melanocyte boundaries of zebrafish and human melanoma. CRATERs are rich in antigen-recognition molecules, harboring the highest density of CD8+ T cells in tumors. In zebrafish, CD8+ T cells formed prolonged interactions with melanoma cells within CRATERs, characteristic of antigen recognition. Following immunostimulatory treatment, CRATERs expanded, becoming the major sites of activated CD8+ T cell accumulation and tumor killing. In humans, elevation in CRATER density in biopsies following immune checkpoint blockade (ICB) therapy correlated with a clinical response to therapy. CRATERs are structures that show active tumor killing and may be useful as a diagnostic indicator for immunotherapy success.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


