以吗啡为原料合成去氧吗啡酮的多公斤级单釜Albright-Goldman氧化/醋酸二烯醇生成序列的建立
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
重要的μ-阿片受体拮抗剂如纳洛酮和纳曲酮的合成使用去氧吗啡酮作为关键的后期中间体,它是由罂粟衍生的生物碱奥帕平或泰卡因制造的。然而,使用吗啡作为起始材料将是有利的,因为它更便宜,更丰富。在本文中,我们描述了吗啡转化为醋酸二烯醇的安装所需的C14羟基的去甲氧吗啡酮。我们的方法比以前描述的方法更有效,并使用一锅Albright-Goldman氧化/二烯丙醇醋酸酯形成序列作为主要特征。这种合成方法已成功地应用于数公斤尺度。对其他底物上的一锅Albright-Goldman氧化/二烯醇酯形成的范围进行了简要的研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
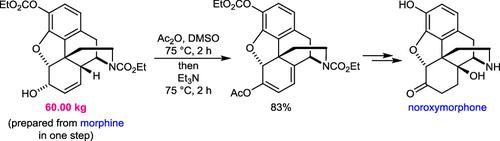
Development of a Multikilogram-Scale One-Pot Albright–Goldman Oxidation/Dienol Acetate Formation Sequence for the Synthesis of Noroxymorphone from Morphine
The syntheses of therapeutically important μ-opioid receptor antagonists such as naloxone and naltrexone use noroxymorphone as a key late-stage intermediate, which is manufactured from the poppy-derived alkaloids oripavine or thebaine. However, it would be advantageous to instead use morphine as the starting material, which is cheaper and more abundant. In this paper, we describe the conversion of morphine into a dienol acetate required for installation of the C14 hydroxyl group of noroxymorphone. Our approach is more efficient than previously described approaches and uses a one-pot Albright–Goldman oxidation/dienol acetate formation sequence of an allylic alcohol as the key feature. This synthesis has been successfully applied on multikilogram scales. A brief investigation of the scope of the one-pot Albright–Goldman oxidation/dienol ester formation on other substrates is also described.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


