高效液相色谱-质谱法准确测定高含量N,N-二甲基甲酰胺药品中N-亚硝基二甲胺
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
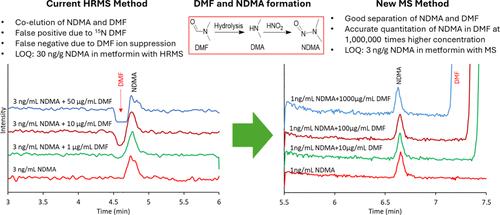
药品中亚硝胺的存在给制药行业带来了重大挑战,主要是因为它们对患者构成潜在的致癌风险。其中,n -亚硝基二甲胺(NDMA)作为最早在缬沙坦、雷尼替丁和二甲双胍等产品中发现的亚硝胺之一引起了人们的关注。NDMA是一种极性化合物,在大多数固定相上具有弱保留,使其容易受到基质干扰。一个普遍的问题是NDMA和N,N-二甲基甲酰胺(DMF)的共溶,DMF是NDMA的常见溶剂和前体。DMF通常以更高的浓度存在,由于15N DMF和13C DMF的干扰,这可能导致假阳性和NDMA浓度的高估。相反,DMF浓度升高可诱导离子抑制,导致假阴性。因此,即使使用高分辨率或串联质谱技术,准确定量NDMA仍然是一个挑战。为了解决这些问题,我们开发了一种强大的HPLC-MS方法,采用Evosphere AQUA柱,可以很好地从DMF和其他样品基质中分离NDMA。该方法允许在DMF存在的情况下精确定量NDMA,其浓度高达1,000,000倍。我们使用单一四极杆质谱仪(如QDa)获得了0.3 ng/mL的定量限,相对于100 mg/mL二甲双胍HCl样品浓度,对应于3 ng/g NDMA,低于NDMA可接受摄入量的10% (32 ng/g)。该方法已根据ICH指南成功验证,证明了特异性、敏感性、准确性、精密度和鲁棒性。通过对二甲双胍制剂(包括速释和缓释制剂)中NDMA的分析,进一步说明了该方法的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Accurate Quantitation of N-Nitrosodimethylamine in Pharmaceutical Products with High Levels of N,N-Dimethylformamide by HPLC-MS
The presence of nitrosamines in pharmaceutical products presents significant challenges for the industry, primarily due to the potential carcinogenic risks they pose to patients. Among these, N-nitrosodimethylamine (NDMA) has garnered attention as one of the first nitrosamines identified in products such as Valsartan, Ranitidine, and Metformin. NDMA is a polar compound with weak retention on most stationary phases, making it susceptible to matrix interferences. A prevalent issue is the coelution of NDMA and N,N-dimethylformamide (DMF), a common solvent and precursor for NDMA. DMF is typically present in much higher concentrations, which can lead to false positives and an overestimation of NDMA concentration due to interference from 15N DMF and 13C DMF. Conversely, elevated DMF concentrations can induce ion suppression, resulting in false negatives. Consequently, accurately quantifying NDMA remains a challenge even when utilizing high-resolution or tandem mass spectrometry techniques. To address these issues, we developed a robust HPLC-MS method employing an Evosphere AQUA column, which enables the good separation of NDMA from DMF and other sample matrices. This method permits accurate quantification of NDMA in the presence of DMF at concentrations up to 1,000,000 times greater. We achieved a quantitation limit of 0.3 ng/mL using a single quadrupole mass spectrometer, such as QDa, which corresponds to 3 ng/g NDMA relative to a 100 mg/mL Metformin HCl sample concentration, less than 10% acceptable intake of NDMA (32 ng/g). The method has been successfully validated according to ICH guidelines, demonstrating specificity, sensitivity, accuracy, precision, and robustness. The application of this method was further illustrated through the analysis of NDMA in Metformin drug products, including both immediate-release and extended-release formulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


