hbx诱导的HSPB1是一个潜在的治疗靶点,因为它调节HBV cccDNA和肝脏免疫反应
IF 33
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景:慢性乙型肝炎病毒(HBV)感染仍然是一个重大的公共卫生问题,目前的治疗方法不足。在这里,我们报道了hbx升高的热休克蛋白B1 (HSPB1)有助于HBV共价闭合环状DNA (cccDNA)的调节和肝脏免疫反应。方法采用体外分子和细胞实验研究HSPB1对HBV感染细胞中HBV cccDNA和HBV复制的影响及其机制。在hbv感染细胞、人肝嵌合小鼠和基于水动力注射的hbv持续免疫活性C57BL/6小鼠中,评估了靶向cccDNA的候选药物的抗病毒效果和免疫应答。结果shspb1通过减少APOBEC3A/APOBEC3B对cccDNA的募集和结合,增强了cccDNA的稳定性。与HBx的相互作用通过降低其泛素化导致HSPB1细胞水平升高,并通过其增强的磷酸化促进HSPB1的核积累。在治疗方面,我们开发了一种肽D-TK,来源于HBXIP肽序列,其靶向HBx并通过阻断HSPB1和HBx之间的相互作用来限制cccDNA,最终导致HBV复制减少。D-TK通过HSPB1降低调节性T (Treg)细胞的频率,增强CD8+ T细胞的频率和活性,从而有助于抑制HBV复制。结论shspb1通过调节HBV cccDNA和肝脏免疫应答,是一种新的治疗靶点。D-TK是一种通过抑制cccDNA和免疫激活来治疗HBV的有前景的临床候选药物。影响和意义慢性乙肝病毒感染仍然是一个重大的全球健康问题,目前的治疗方案是不足的。在这项研究中,我们证明了hbx诱导的HSPB1升高有助于HBV cccDNA和肝脏免疫反应的调节。我们设计了一种肽D-TK,其靶向HBx,有效抑制cccDNA,并通过HSPB1激活免疫反应。我们的研究结果为hbx诱导的HSPB1调节HBV cccDNA和肝脏免疫调节的机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
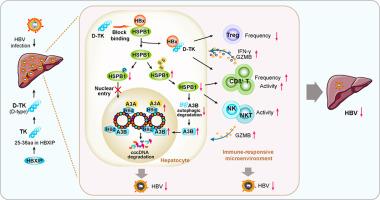
HBx-induced HSPB1 is a potential therapeutic target owing to its modulation of HBV cccDNA and hepatic immune responses
Background & Aims
Chronic infection with hepatitis B virus (HBV) remains a significant public health concern, and current therapies are inadequate. Here, we report that HBx-elevated heat shock protein B1 (HSPB1) contributes to the regulation of HBV covalently closed circular DNA (cccDNA) and the hepatic immune responses.Methods
Molecular and cellular in vitro assays were employed to investigate the effects and underlying mechanisms of HSPB1 on HBV cccDNA and HBV replication in HBV-infected cells. The anti-viral efficacy of candidate agents targeting cccDNA and the immune response was assessed in HBV-infected cells, human-liver chimeric mice, and hydrodynamic injection-based HBV-persistent immunocompetent C57BL/6 mice.Results
HSPB1 enhanced cccDNA stability by reducing the recruitment and binding of APOBEC3A/APOBEC3B to cccDNA. Interaction with HBx led to increased cellular levels of HSPB1 by decreasing its ubiquitination, and nuclear accumulation of HSPB1 was promoted via its enhanced phosphorylation. Therapeutically, we developed a peptide, D-TK, derived from HBXIP peptide sequence that targets HBx and limits cccDNA by blocking the interaction between HSPB1 and HBx, ultimately resulting in reduced HBV replication. Nobly, D-TK reduced the frequency of regulatory T (Treg) cells and enhanced the frequency and activity of CD8+ T cells through HSPB1, thereby contributing to suppression of HBV replication.Conclusions
HSPB1 represents a novel therapeutic target by modulating HBV cccDNA and immune responses in the liver. D-TK is a promising clinical candidate for HBV treatment through cccDNA suppression and immune activation.Impact and Implications
Chronic infection with HBV remains a significant global health concern, and current therapeutic options are inadequate. In this study, we demonstrate that HBx-induced elevation of HSPB1 contributes to the regulation of HBV cccDNA and the hepatic immune responses. We engineered a peptide, D-TK, that targets HBx, effectively suppresses cccDNA, and activates immune responses through HSPB1. Our findings offer new insights into the mechanism by which HBx-induced HSPB1 modulates HBV cccDNA and immune regulation in the liver.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


