亚砜亚胺辅助非活化烯烃δ-1,1-级联杂环化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
该研究证明了钯通过C(烯基)-H活化、氧化加成/还原消除和同步核金属化的组合,催化了未活化烯烃的δ-环结1,1-二功能化。2-吡啶基甲基亚砜(MPyS)双齿导向基团与2-羟基-5-甲基吡啶配体是在温和条件下实现转化的关键。该工艺可以在一次操作中形成C-C / C-O键,使用2-碘苯酰胺或2-碘苯甲酸系列的双功能试剂(BFRs)证明了这一点。值得注意的是,重新合成α-支化异苯并呋喃-1(3H)-亚胺和邻苯二甲酸盐强调了它的实用性。机理研究和DFT计算揭示了催化循环的复杂性。此外,该方法还提供了直接合成单胺氧化酶a抑制剂的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
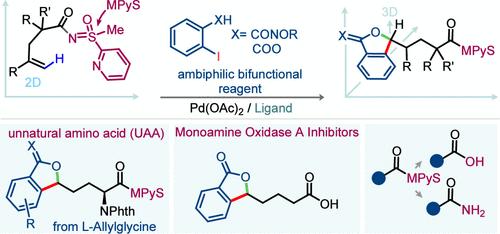
Sulfoximine Aided δ-1,1-Cascade Heterocyclization of Unactivated Alkenes
This study demonstrates a palladium-catalyzed δ-annulative 1,1-difunctionalization of unactivated alkenes through a combination of C(alkenyl)–H activation, oxidative addition/reductive elimination, and syn-nucleometalation. The 2-pyridyl-methyl sulfoximine (MPyS) bidentate directing group, together with the 2-hydroxy-5-methylpyridine ligand, is crucial for making the transformation feasible under mild conditions. The process enables the formation of C–C/C–O bonds in a single operation, as evidenced by the use of bifunctional reagents (BFRs) of the 2-iodobenzamide or 2-iodobenzoic acid series. Notably, the de novo synthesis of α-branched isobenzofuran-1(3H)-imine and phthalides underscores its practical utility. Mechanistic studies and DFT computations uncover the intricacies of the catalytic cycle. Additionally, this method provides direct access to the synthesis of a monoamine oxidase A inhibitor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


