具有耐酸微环境的腔网络铜纳米催化剂用于高效CO2电还原乙烯
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
亲气/疏水微结构Cu纳米材料解决了电催化CO2还原反应(CO2RR)中多碳(C2+)选择性瓶颈,但其形态控制受到低还原电位和高表面原子迁移率的阻碍。本研究报告了一种生物气捕集疏水Cu纳米结构(BGH-Cu),通过原位电化学重建,形成一个相互连接的腔网络,赋予亲气/疏水特性。机理研究表明,空腔作为动态电解质储存器,选择性保留OH−/K+以抑制质子输运并创造局部微碱性环境,而空腔增强的阳离子富集与C─C耦合协同作用。在强酸性条件下(pH 1),电流密度为600 mA cm−2时,BGH-Cu的C2H4法拉第效率为54.7%,单次碳效率为63.7%,膜电极结构稳定性为40 h。本研究为亲气/疏水Cu纳米材料提供了一种非极端合成策略,阐明了C2+选择性的“腔微环境调制”机制,并为高电流密度酸性CO2RR到C2+产物提供了新的范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
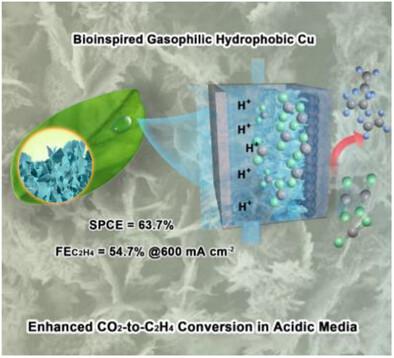
Cavity-Networked Copper Nanocatalysts with Acid-Tolerant Microenvironments for Efficient CO2 Electroreduction to Ethylene
Gasophilic/hydrophobic microstructured Cu nanomaterials address the multi-carbon (C2+) selectivity bottleneck in electrocatalytic CO2 reduction reaction (CO2RR), yet their morphological control is hindered by low reduction potentials and high surface atomic mobility. This study reports a bioinspired gas-trapping hydrophobic Cu nanostructure (BGH-Cu) via in situ electrochemical reconstruction, forming an interconnected cavity network that confers gasophilic/hydrophobic properties. Mechanistic studies reveal cavities act as dynamic electrolyte reservoirs, selectively retaining OH−/K+ to suppress proton transport and create a local micro-alkaline environment, while cavity-enhanced cation enrichment synergizes with C─C coupling. BGH-Cu achieves C2H4 Faradaic efficiency of 54.7% at current density of 600 mA cm−2 in strongly acidic conditions (pH 1), with 63.7% single-pass carbon efficiency and 40 h stability in a membrane electrode assembly configuration. This work provides a non-extreme synthesis strategy for gasophilic/hydrophobic Cu nanomaterials, elucidates the “cavity microenvironment modulation” mechanism for C2+ selectivity, and offers a new paradigm for high-current-density acidic CO2RR to C2+ products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


