在颗粒、转录物和蛋白质水平上靶向和跟踪mRNA脂质纳米颗粒。
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
脂质纳米颗粒(LNPs)是一个多功能平台,具有众多的实验,诊断和治疗应用。然而,LNPs在肝细胞和抗原提呈细胞中积聚的高倾向在很大程度上限制了它们在疫苗和肝脏疾病治疗中的临床应用。在这篇综述中,我们描述了用于监测rna负载LNPs (RNA-LNPs)的体内行为的成熟和新兴技术,以及改进组织和细胞靶向的策略,以扩大该技术的应用。我们讨论了LNP化学和生物工程方面的创新,以及利用合成生物学来定制mRNA货物的细胞表达,包括使用microRNA靶点。我们还回顾了如何修改编码蛋白来控制负载的稳定性和亚细胞定位。最后,我们讨论了这些技术和策略如何加速RNA-LNP药物的临床翻译和改善治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
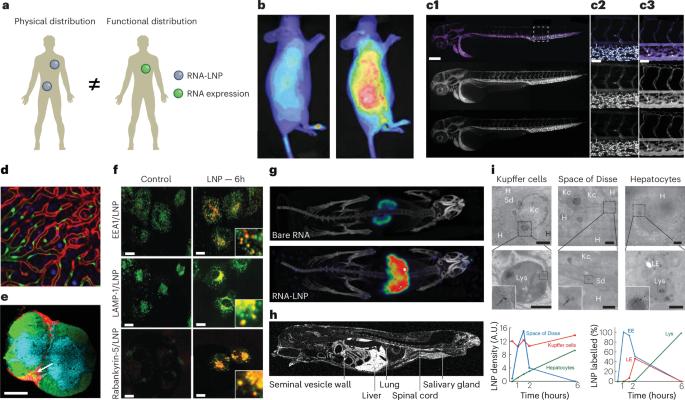
Targeting and tracking mRNA lipid nanoparticles at the particle, transcript and protein level
Lipid nanoparticles (LNPs) are a versatile platform with numerous experimental, diagnostic and therapeutic applications. However, the high propensity of LNPs to accumulate in hepatocytes and antigen-presenting cells has largely limited their clinical use to vaccines and the treatment of liver diseases. In this Review, we describe established and emerging techniques for monitoring the in vivo behaviour of RNA-loaded LNPs (RNA-LNPs) and strategies for improved tissue and cell targeting that expand the applications of this technology. We discuss innovations in LNP chemistry and bioengineering, and exploiting synthetic biology to tailor the cellular expression of the mRNA cargo, including the use of microRNA target sites. We also review how modifications to the encoded protein can be used to control stability and subcellular localization of the payload. Lastly, we discuss how these techniques and strategies can accelerate the clinical translation of RNA-LNP drugs and improve therapeutic outcomes. This Review explores strategies for targeting and tracking RNA-LNP drugs, monitoring their in vivo behaviour, enhancing tissue-specific delivery, and using synthetic biology to control mRNA expression and protein localization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


