手性铜-氨基酸配体络合物催化2-萘酚的不对称氧化偶联
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种[Cu(CH3CN)4]+BF4 - /空气介导的不对称偶联方法,使用新型l-氨基酸衍生的氨基吡啶酰胺配体来接触各种C2/ c1对称的binol。这是含酰胺配体在铜催化2-萘酚不对称氧化偶联中的首次应用。值得注意的是,酯交换和偶联过程的高效一锅整合为合成酯功能化BINOLs建立了一种新的策略。在单晶x射线衍射分析中,Cu(I)组分中发现了高活性Cu2O3氧化物的形成,这是立体选择控制催化循环的关键决定因素。此外,该方法还可以有效地合成外消旋双萘洛酮化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
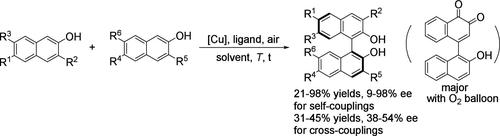
Asymmetric Oxidative Coupling of 2-Naphthols Catalyzed by Chiral Copper–Amino Acid-Derived Ligand Complexes
We developed a [Cu(CH3CN)4]+BF4–/air-mediated asymmetric coupling method using novel l-amino acid-derived aminopyridine–amide ligands to access various C2/C1-symmetric BINOLs. This represents the first application of amide-containing ligands in copper-catalyzed asymmetric oxidative coupling of 2-naphthols. Notably, the efficient one-pot integration of the transesterification and coupling processes establishes a novel strategy for synthesizing ester-functionalized BINOLs. The formation of highly active Cu2O3 oxides was detected from Cu(I) species under single-crystal X-ray diffraction analysis and is the key determinant for the stereoselective control of the catalytic cycle. Moreover, this method also enables the efficient synthesis of racemic binaphtholone compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


