重新设计电压门控阴离子通道抑制神经元放电
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
设计响应环境信号的离子通道对调节细胞活动和传感器的发展具有重要意义,但由于设计刺激诱导的蛋白质构象变化的复杂性,它仍然是一个重大挑战。在这里,我们报道了电压门控阴离子通道(dVGACs)的精确从头设计。在膜片钳实验中,dVGACs采用15螺旋五聚体结构,在跨膜范围内存在精氨酸收缩,并显示出电压依赖的阴离子电流。低温电子显微镜(cryo-EM)显示,dVGACs的结构与设计模型非常吻合。低温电镜结构和分子动力学模拟表明,精氨酸收缩体经历电压诱导的构象变化,既可以作为电压传感器,又可以作为选择性滤波器。值得注意的是,dVGACs的阴离子选择性和电压敏感性可以通过靶向突变来原位抑制神经元放电。通过定制设计的构象变化创建离子通道的能力刷新了我们对膜生物物理学的见解,并揭示了各种潜在的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
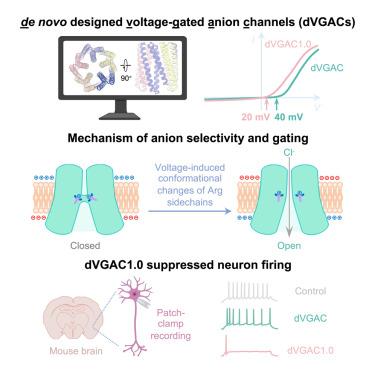
De novo designed voltage-gated anion channels suppress neuron firing
Design of ion channels responsive to environmental cues has significant implications in modulating cellular activities and sensor development, but it remains a significant challenge due to the complexities involved in designing stimuli-induced conformational changes in proteins. Here, we report the accurate de novo design of voltage-gated anion channels, namely dVGACs. dVGACs adopt a 15-helix pentameric architecture featuring arginine constrictions within the transmembrane span and show voltage-dependent anions currents in patch-clamp experiments. Cryo-electron microscopy (cryo-EM) structures of dVGACs closely align with the design models. Cryo-EM structures and molecular dynamics simulations suggest that the arginine constrictions undergo voltage-induced conformational changes, serving as both a voltage sensor and a selectivity filter as designed. Notably, the anion selectivity and voltage sensitivity of dVGACs can be tuned through targeted mutations for suppressing neuronal firing in situ. The ability to create ion channels with custom-designed conformational changes refreshes our insights into membrane biophysics and unveils diverse potential applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


