Janus氧化石墨烯纳米平台具有相反功能的粘附和润滑,使局部持续的非诺贝特释放协同抑制骨关节炎
IF 26.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
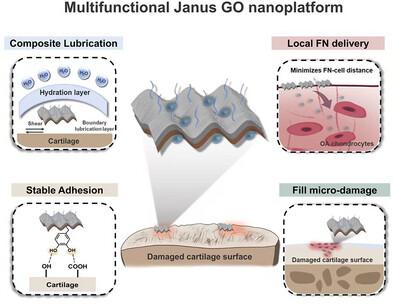
骨关节炎(OA)的进展是一个包括软骨损伤、摩擦、润滑损失和软骨细胞衰老的破坏性循环。目前的治疗仅限于暂时润滑或缓解疼痛,由于无法修复软骨或恢复先天润滑,无法阻止OA。为了解决这一问题,研究人员设计了一种不对称的Janus氧化石墨烯(MGO)纳米平台,并使用抗衰老剂非诺贝特(FN)进行功能化,形成了MGO-FN系统。这种集成设计的特点是一侧提供强大的软骨粘附,另一侧提供卓越的润滑,同时提供治疗性FN。至关重要的是,纳米级的MGO-FN有效地渗透并填充软骨表面的微损伤,从而实现局部和持续的FN释放。通过最小化扩散距离,使药物在靶点的生物利用度最大化。在体外,MGO-FN表现出强大的协同作用,显著增强软骨细胞增殖和细胞外基质合成,减少衰老,并比单独使用任何一种成分更有效地上调润滑标志物PRG4。在转录组学分析的支持下,OA大鼠体内研究证实了MGO-FN的有效治疗效果,包括减少软骨降解、减轻炎症、促进基质再生和恢复先天润滑。这些发现强调了MGO-FN作为一种有希望的多方面治疗策略,通过同时恢复软骨完整性和润滑功能来阻止OA进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Janus Graphene Oxide Nanoplatform with Oppositely Functionalized Adhesion and Lubrication Enables Local Sustained Fenofibrate Release to Synergistically Halt Osteoarthritis
Osteoarthritis (OA) progresses via a destructive cycle involving cartilage damage, friction, lubrication loss, and chondrocyte senescence. Current therapies, limited to temporary lubrication or pain relief, fail to halt OA due to their inability to repair cartilage or restore innate lubrication. To address this challenge, an asymmetric Janus graphene oxide (MGO) nanoplatform is engineered and functionalized with the anti-senescence agent Fenofibrate (FN), creating the MGO-FN system. This integrated design features one side providing robust cartilage adhesion and the opposing side offering superior lubrication, while simultaneously delivering the therapeutic FN. Critically, the nanoscale MGO-FN effectively infiltrates and fills micro-damage on the cartilage surface, enabling localized and sustained FN release. This maximizes drug bioavailability at the target site by minimizing diffusion distances. In vitro, MGO-FN demonstrated potent synergistic effects, significantly enhancing chondrocyte proliferation and extracellular matrix synthesis, reducing senescence, and upregulating the lubrication marker PRG4 more effectively than either component alone. In vivo OA rat studies, supported by transcriptomics analysis, validated MGO-FN's potent therapeutic effects, including reduced cartilage degradation, mitigated inflammation, promoted matrix regeneration, and restored innate lubrication. These findings underscore MGO-FN as a promising multifaceted therapeutic strategy to halt OA progression by concurrently restoring cartilage integrity and lubricating function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


