具有模拟dna酶活性和自供应H2O2能力的工程多功能纳米平台,用于增强化学动力学生物膜根除
IF 14.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
生物膜感染是一种严重的全球健康威胁,其特点是死亡率高,对常规疗法有很大的耐药性。化学动力疗法(CDT)利用Fenton或Fenton样反应产生杀菌羟基自由基(•OH),已成为对抗浮游细菌感染的一种很有前途的方法。然而,由于保护性的细胞外聚合物(EPS)基质和较低的内源性H2O2浓度,其对生物膜的有效性仍然有限。为了克服这些挑战,研究人员开发了一种级联激活的纳米平台,将模拟脱氧核糖核酸酶的成分与自我维持的H2O2生成结合起来。该平台由Ce4+/nitrilotriacetic acid (NTA)配合物组成,这些配合物固定在包封CuO2纳米点的氨基功能化SiO2壳上。在生物膜的酸性微环境中,这种纳米平台启动了双管齐下的方法:首先,Ce4+/NTA复合物选择性地降解EPS基质内的细胞外DNA,破坏生物膜结构并促进更深的渗透;其次,CuO2分解释放大量H2O2和Cu2+离子,后者催化芬顿样反应,将H2O2转化为细胞毒性•OH自由基,诱导细菌膜脂过氧化。该组合策略在体外表现出出色的抗生物膜性能,去除99.9%以上的金黄色葡萄球菌(S. aureus)和铜绿假单胞菌(P. aeruginosa)生物膜。使用金黄色葡萄球菌感染的小鼠伤口模型进行的体内测试表明,细菌定植显著减少,同时组织修复动力学增强,没有可检测到的毒理学效应。通过同时克服基质穿透障碍和自主生成H2O2,该方法可以增强CDT对抗持续性生物膜感染的功效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
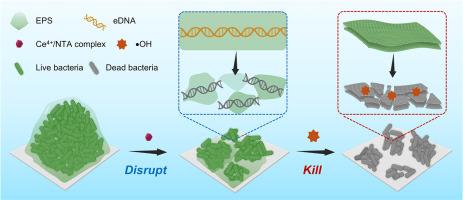
Engineered multifunctional nanoplatform with DNase-mimetic activity and self-supplying H2O2 capability for enhanced chemodynamic biofilm eradication
Biofilm infections represent a critical global health threat, characterized by high mortality and significant resistance to conventional therapies. Chemodynamic therapy (CDT), which harnesses Fenton or Fenton-like reactions to generate bactericidal hydroxyl radicals (•OH), has emerged as a promising approach for combating planktonic bacterial infections. Nevertheless, its effectiveness against biofilms remains limited because of the protective extracellular polymeric substance (EPS) matrix and low endogenous H2O2 concentrations. To overcome these challenges, a cascade-activatable nanoplatform was developed, integrating deoxyribonuclease-mimetic components with self-sustaining H2O2 generation. The platform consists of Ce4+/nitrilotriacetic acid (NTA) complexes immobilized on amino-functionalized SiO2 shells encapsulating CuO2 nanodots. In the acidic microenvironment of biofilms, this nanoplatform initiates a two-pronged approach: First, the Ce4+/NTA complexes selectively degrade extracellular DNA within the EPS matrix, disrupting biofilm structure and facilitating deeper penetration; Second, CuO2 decomposition releases substantial H2O2 and Cu2+ ions, the latter catalyzing a Fenton-like reaction that converts H2O2 into cytotoxic •OH radicals, inducing bacterial membrane lipid peroxidation. This combined strategy demonstrated outstanding antibiofilm performance in vitro, eliminating over 99.9% of both Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa)biofilms. In vivo testing using an S. aureus-infected murine wound model demonstrated a substantial decrease in bacterial colonization alongside enhanced tissue repair kinetics, with no detectable toxicological effects. By simultaneously overcoming matrix penetration barriers and autonomously generating H2O2, this approach offers a robust enhancement of CDT efficacy against persistent biofilm infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


