强化CO2电还原用二元纳米合金中的金原子比:AgPd的双金属协同作用
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
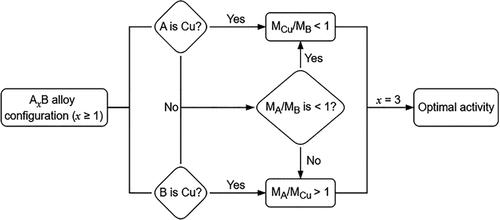
在各种用于电化学CO2还原反应(CO2RR)的二元纳米合金中观察到一个有趣的现象,其中原子比接近1:3的情况下大多产生最佳活性,但其起源仍然知之甚少。在这里,我们合成了一系列具有精确控制原子比的AgxPd1-x纳米团簇(nc)作为模型系统,以验证其普遍性和内在推导性,因为Ag具有高的内在CO选择性,但由于中间结合弱,需要大的过电位(η),而Pd以小η形成CO,但由于CO吸附太强而遭受CO中毒。事实上,原子比为1:3的Ag0.25Pd0.75 NCs具有最佳的CO2RR活性,提供接近100%的CO法拉第效率和最高71.8%的能量效率。计算表明,银/钯原子比为1:3时,产生了相对于费米能级有利的d带中心的最佳电子结构。这种结构协同降低了*COOH形成的能势,促进了*CO的解吸动力学,原位光谱分析证实了这一点,与其他化学统计相比,Ag0.25Pd0.75 NCs的*CO吸附信号减弱,表明CO的解吸能力增强。该研究为CO2RR纳米合金的“黄金比例”提供了深入的机理见解,并揭示了设计先进纳米合金的通用范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Golden Atomic Ratio in Binary Nanoalloys for Enhanced CO2 Electroreduction: Dual-Metal Synergy of AgPd
An intriguing phenomenon has been observed in various binary nanoalloys for the electrochemical CO2 reduction reaction (CO2RR), where an atomic ratio close to 1:3 mostly yields the optimal activity, but its origin remains poorly understood. Here, we synthesized a series of size-uniform AgxPd1–x nanoclusters (NCs) with precisely controlled atomic ratios as a model system to verify its universality and intrinsic derivation since Ag offers a high intrinsic CO selectivity but requires a large overpotential (η) due to weak intermediate binding, while Pd forms CO at a small η but suffers from CO poisoning due to an overly strong CO adsorption. Indeed, Ag0.25Pd0.75 NCs with an atomic ratio of 1:3 possessed optimal CO2RR activity, delivering a nearly 100% CO Faraday efficiency and a maximum energy efficiency of 71.8%. Computational calculations demonstrated that the Ag/Pd atomic ratio of 1:3 induced an optimal electronic structure characterized by a d-band center positioned favorably relative to the Fermi level. This configuration synergistically lowered the energy barrier for *COOH formation and promoted *CO desorption kinetics, as corroborated by in situ spectroscopic analysis, where Ag0.25Pd0.75 NCs exhibited attenuated *CO adsorption signals compared with other stoichiometries, indicating enhanced CO desorption capability. This study provides in-depth mechanistic insights into the “golden ratio” in nanoalloys for the CO2RR and reveals a universal paradigm of designing advanced nanoalloys.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


