利培酮通过smpd1溶酶体介导的铁下垂诱导骨质疏松和神经精神治疗抵抗:活性维生素D类似物ED-71的双重拯救
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
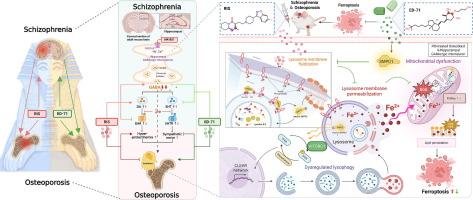
利培酮(RIS)是第二代精神分裂症(SZ)抗精神病药,与骨质疏松症和次优症状缓解有关。这些跨组织毒性(骨和海马体)的共同细胞机制仍然不明确,阻碍了治疗的进展。目的确定RIS是否通过酸性鞘磷脂酶(SMPD1)-溶酶体轴诱导成骨细胞和海马gaba能神经元的毒性,并评估活性维生素D类似物eldecalcitol (ED-71)减轻这些作用的治疗潜力。方法采用二唑西平诱导的SZ小鼠进行骨质流失和精神症状评价。用RIS处理骨髓间充质干细胞(BMSCs)和MC3T3-E1成骨细胞诱导的原代成骨细胞,以及海马原代神经元和HT22海马神经元,以评估铁凋亡、溶酶体功能障碍和SMPD1活性。关键技术包括微型ct、组织形态学染色、行为测试、酶联免疫吸附测定、RNA测序、靶向脂质组学分析、分子动力学模拟和等温滴定量热测定。虚拟药物筛选用于鉴定潜在的RIS-SMPD1相互作用拮抗剂,随后对鉴定的候选ED-71进行进一步验证。结果ris靶向溶酶体,引起成骨细胞和海马gaba能神经元的功能障碍和膜通透性,导致铁细胞凋亡。从机制上讲,RIS在ARG294位点与SMPD1结合,抑制其活性并引发溶酶体磷脂积累和铁下沉。在SZ模型中,ED-71破坏RIS- smpd1相互作用,恢复溶酶体完整性,减轻高泌乳素血症/交感神经过度激活,并与RIS协同增强抗精神病疗效和预防骨质疏松症。结论本研究确定SMPD1激活可作为对抗ris诱导的骨和海马铁下垂的治疗靶点。ED-71的双重作用机制为SZ干预提供了一种新的策略,同时解决精神症状和骨质疏松症并发症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Risperidone induces osteoporosis and neuropsychiatric treatment resistance via SMPD1-lysosome-mediated ferroptosis: dual rescue by active vitamin D analog ED-71
Introduction
Risperidone (RIS), a second-generation antipsychotic for schizophrenia (SZ), is linked to osteoporosis and suboptimal symptom resolution. The shared cellular mechanisms underlying these cross-tissue toxicities (bone and hippocampus) remain undefined, hindering therapeutic advancement.Objectives
To determine if RIS induces toxicity in osteoblasts and hippocampal GABAergic neurons via the acid sphingomyelinase (SMPD1)-lysosome axis, and to evaluate the therapeutic potential of eldecalcitol (ED-71), an active vitamin D analog, in mitigating these effects.Methods
Dizocilpine-induced SZ mice were used to evaluate bone loss and psychiatric symptoms. Primary osteoblasts induced from bone marrow mesenchymal stem cells (BMSCs) and MC3T3-E1 osteoblasts, as well as primary hippocampal neurons and HT22 hippocampal neurons, were treated with RIS to evaluate ferroptosis, lysosomal dysfunction, and SMPD1 activity. Key techniques included micro-CT, histomorphometric staining, behavioral tests, enzyme-linked immunosorbent assay, RNA sequencing, targeted lipidomics analysis, molecular dynamics simulations, and isothermal titration calorimetry assays. Virtual drug screening was used to identify potential RIS-SMPD1 interaction antagonists, with the identified candidate ED-71 further validated thereafter.Results
RIS targeted lysosomes, causing dysfunction and membrane permeabilization, which drove ferroptosis in osteoblasts and hippocampal GABAergic neurons. Mechanistically, RIS bound to SMPD1 at ARG294, inhibiting its activity and triggering lysosomal phospholipid accumulation and ferroptosis. ED-71 disrupted RIS-SMPD1 interactions, restored lysosomal integrity, mitigated hyperprolactinemia/sympathetic overactivation, and synergized with RIS to enhance antipsychotic efficacy and prevent osteoporosis in SZ models.Conclusion
This study identifies SMPD1 activation as a therapeutic target to counteract RIS-induced ferroptosis in bone and hippocampus. The dual-action mechanism of ED-71 provides a novel strategy for SZ intervention, simultaneously addressing psychiatric symptoms and osteoporotic complications.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


