通过3-吡啶磺酸的溶剂化护套重构和氢键网络调节协同设计长寿命水性锌电池
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由于其固有的安全性和成本效益,水性锌离子电池(azib)正在成为大规模储能系统的有前途的候选者。然而,由于树突晶体的快速生长和水电解质中大量活性水分子分解引起的严重副反应,极大地阻碍了它们的实际应用。在本研究中,以最佳浓度引入3-吡啶磺酸(3-PSA)分子作为azib的电解质添加剂。通过电化学表征和理论计算相结合,发现磺酸基(−SO3H)与Zn2+之间的定向配位相互作用导致(Zn(H2O)6)2+的溶剂化壳层重组,吸附在Zn金属表面,促进了Zn2+的均匀沉积,并显著减少了游离H2O分子的数量。此外,3-PSA的引入破坏了原有的氢键网络,调整了氢键键角,抑制了水腐蚀和副反应。使用50 mM 3-PSA/ m2 ZnSO4电解液的Zn//Zn对称电池在1 mA cm-2 /1 mAh cm-2条件下具有优异的循环寿命(2700 h),而Zn//MnO2全电池在2 A g-1条件下可达到1500次超稳定循环,显著优于使用纯ZnSO4电解液的对照组。该研究为高性能AZIB电解质的工程设计提供了新的思路和策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
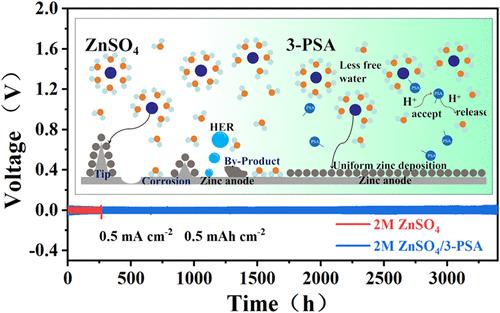
Synergistic Design of Long-Life Aqueous Zinc Batteries Through Solvation Sheath Reconstruction and Hydrogen-Bond Network Regulation by 3-Pyridinesulfonic Acid
Aqueous zinc-ion batteries (AZIBs) are emerging as a promising candidate for large-scale energy storage systems due to their inherent safety and cost-effectiveness. However, the vigorous growth of dendritic crystals and severe side reactions caused by the decomposition of a large number of active water molecules in aqueous electrolytes significantly hinder their practical application. In this study, 3-pyridinesulfonic acid (3-PSA) molecules were introduced at an optimized concentration as an electrolyte additive for AZIBs. Through a combination of electrochemical characterization and theoretical calculations, it was found that the directed coordination interaction between the sulfonic acid group (−SO3H) and Zn2+ leads to the restructuring of the solvation shell of (Zn(H2O)6)2+, which adsorbs onto the Zn metal surface, promoting uniform Zn2+ deposition and significantly reducing the number of free H2O molecules. Additionally, the introduction of 3-PSA disrupts the original hydrogen bond network, adjusts the hydrogen bond bond angles, and suppresses water-induced corrosion and side reactions. The Zn//Zn symmetric cell using the designed 50 mM 3-PSA/2 M ZnSO4 electrolyte demonstrated excellent cycle life (2700 h) under 1 mA cm–2/1 mAh cm–2 conditions, while the Zn//MnO2 full cell achieved 1500 ultrastable cycles under 2 A g–1 conditions, significantly outperforming the control group using pure ZnSO4 electrolyte. This study provides new insights and strategies for the engineering design of high-performance AZIB electrolytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


