氨煤共燃烧过程中NO还原的机理研究:煤焦表面官能团的DFT研究
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
煤燃烧产生的烟气中的氮氧化物(NOx)会造成严重的环境污染,而氨被认为是一种很有前途的零碳能源。然而,氨也有局限性,包括低可燃性和氮氧化物排放。氨煤共燃技术是一种主流选择。相关研究表明,氧气的存在可以通过产生新的活性位点来帮助减少NO。以往对氨煤共燃技术结合空气分级燃烧的研究主要是基于实验研究,缺乏对反应机理的探索。因此,我们利用密度泛函理论(DFT)来探讨悬空键、C=O和- OH基团对炭的反应活性、NO还原途径和反应动力学的影响。结果表明,Zig@OH模型对NO的吸附能力最强。-OH和C=O基团都有利于缩短反应途径,促进N-N结构的形成。因此,O2促进了氨与煤的共燃,这与实验结果一致。Zig@O模型具有最低的速率决定阶跃能垒。三种模型的定速阶能垒总体上没有显著差异。动力学计算表明,在800 ~ 1600 K的温度范围内,三种含氨炭化模式均能有效促进NO的还原。本研究旨在为氨煤共燃技术中NO/O2/NH3-char反应的微观机理提供新的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
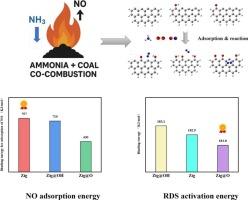
Mechanistic insights into NO reduction during ammonia-coal co-combustion: A DFT study on char surface functional groups
Nitrogen oxides (NOx) in flue gas generated by coal combustion can cause severe environmental pollution, and ammonia is regarded as a promising zero-carbon energy source. However, ammonia also has limitations, including low flammability and NOx emissions. Ammonia-coal co-combustion technology is a mainstream choice. Relevant research indicates that the presence of oxygen can assist in the reduction of NO by creating new active sites. Previous studies on ammonia-coal co-combustion technology combined with air-staged combustion were mainly based on experimental research, lacking exploration of the reaction mechanism. Therefore, we used density functional theory (DFT) to explore the effects of dangling bonds, C=O, and −OH groups on the reactivity of char, NO reduction pathways, and reaction kinetics. The conclusion indicates that the Zig@OH model has the strongest NO adsorption capacity. Both –OH and C=O groups are beneficial to shortening the reaction pathway and promote the formation of N-N structure. Therefore, O2 promotes the co-combustion of ammonia and coal, which is consistent with the experimental results. The Zig@O model has the lowest rate-determining step energy barrier. The rate-determining step energy barriers of the three models are not significantly different overall. The kinetic calculation illustrated that within the temperature range of 800–1600 K, three char models with ammonia can effectively promote the reduction of NO. This study aims to provide new insights into the microscopic mechanism of the NO/O2/NH3-char reaction in ammonia-coal co-combustion technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


