低氧条件下褐煤中优势反应官能团的响应机制:相相微观结构与能量变化相结合的研究
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
褐煤的高水分和挥发性使其容易自燃。研究氧化过程中的能量变化和相显微组织对防治煤火具有重要意义。因此,本研究通过分析褐煤微观结构特征,结合Pearson相关分析,探讨缺氧条件对褐煤低温氧化特性的影响,以及基团的相机制。结果表明:在脱水脱气阶段,褐煤表面呈颗粒状结构;活化能对O2浓度的敏感性显著提高。O2浓度的降低增强了基团的自反应,限制了褐煤与氧之间的化学吸附。在动态平衡阶段,褐煤表面形成鳞状结构。高温效应扩大了基团的反应途径,而缺氧条件抑制了活性结构和挥发物的形成。相关分析表明-OH和-CH2 /CH3是主要的产热基团,-CHO和-COOH是主要的吸热基团。当O2浓度从5%上升到21%时,低温氧化的主要反应基团为-CH2 /CH3→-OH→-CH2 /CH3。此外,缺氧条件抑制了-OH和-COOH的反应,而对-CHO和-CH2 /CH3的影响很小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
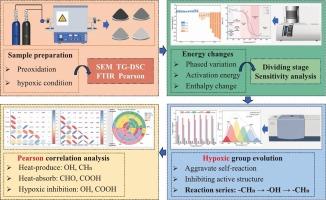
Response mechanism of dominant reactive functional groups in lignite under hypoxic conditions: a study combining phased microstructural and energy changes
High moisture and volatile of lignite make it easy to spontaneous combustion. It is important to study energy changes and phased microstructural in oxidation process for prevention and control of coal fire. Therefore, by analyzing the characteristics of lignite microstructure, combined with Pearson correlation analysis, this study investigates the influence of hypoxic conditions on low-temperature oxidation characteristics of lignite, and phased mechanism of groups. The results indicate that during dehydration and degassing stage, lignite surface exhibits a granular structure. The activation energy exhibits significantly increased sensitivity to O2 concentration. The reduction in O2 concentration intensifies the self-reaction of groups and restricts the chemisorption between lignite and oxygen. During dynamic equilibrium stage, a scale-like structure forms on the lignite surface. The high-temperature effect expands the reaction pathways of groups, while hypoxic condition suppresses the formation of active structures and volatile. Correlation analysis reveals that –OH and –CH2/CH3 are the primary heat-producing groups, and –CHO and –COOH are the primary heat-absorbing groups. When O2 concentration rises from 5 % to 21 %, the main reaction groups of low-temperature oxidation are –CH2/CH3 → –OH → –CH2/CH3. Furthermore, hypoxic condition inhibits the reactions of –OH and –COOH, while exerting minimal influence on –CHO and –CH2/CH3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


