用硫醚作为双功能试剂的反阴离子控制的化学分散转移-氢硫基化/碳硫基化
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
追求能够锻造C(sp3)─S键的催化剂是非常需要的,因为这些键在药物、功能材料和有机合成中具有重要意义。原料烯烃的氢硫基化和碳硫基化是C(sp3)─S键形成的最直接和最突出的方法。然而,烷基取代烯烃的氢硫化通常以反马尔可夫尼科夫方式进行,而碳硫化主要依赖于使用高活性的预功能化亲电性硫源和亲核性碳源的三组分偶联策略。本文中,通过战略性地利用C(sp3)─S键的异裂,我们报道了用硫醚作为双功能试剂的烯烃的化学发散转移-氢硫基化和碳硫基化。化学控制是通过仔细选择与rh中心相关的反阴离子来实现的。具有较强配位能力的反阴离子,如TfO−,促进了不寻常的Markovnikov转移-巯基烯反应。相反,非配位的反阴离子,如BF4−,使分子间的硫醚-烯反应成为可能,直接在烯烃上添加硫醚。机制和计算研究表明,反阴离子在Rh上的配位或不配位可以改变硫代酸盐的碱度,导致化学发散。本文章由计算机程序翻译,如有差异,请以英文原文为准。
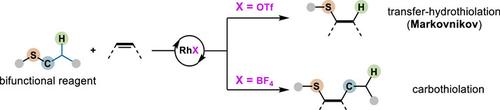
Counteranion-Controlled Chemodivergent Transfer-Hydrothiolation/Carbothiolation Using Thioethers as Bifunctional Reagents
The pursuit of catalysis capable of forging C(sp3)─S bonds is extremely desired because these bonds have substantial importance in pharmaceuticals, functional materials, and organic synthesis. Hydrothiolation and carbothiolation of feedstock alkenes are among the most straightforward and prominent approaches to C(sp3)─S bond formations. However, hydrothiolation of alkyl-substituted alkenes typically proceeds in an anti-Markovnikov fashion, and carbothiolation predominantly relies on three-component coupling strategies using highly reactive pre-functionalized electrophilic sulfur sources and nucleophilic carbon sources. Herein, by strategically using heterolytic cleavage of C(sp3)─S bond, we report a chemodivergent transfer-hydrothiolation and carbothiolation of alkenes with thioethers serving as bifunctional reagents. The chemo-control is achieved through careful selection of the counteranion associated with the Rh-center. Counteranions with relatively strong coordinating ability, such as TfO−, promote an unusual Markovnikov transfer-thiol-ene reaction. Conversely, noncoordinating counteranions, such as BF4−, enable an unprecedented intermolecular thioether-ene reaction that adds thioethers directly across alkenes. Mechanistic and computational studies elucidated that the coordination or noncoordination of counteranions on Rh can alter the basicity of the thiolate, resulting in chemodivergence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


