钯基催化剂促进羟基迁移促进碱性氢氧化
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
pd基材料作为典型的储氢材料,通常具有较强的氢结合能(HBE)和较差的羟基结合能(OHBE),这阻碍了中间体的转移,导致在低电位下氢氧化反应(HOR)活性较差。本文制备了MoOx簇修饰的PdCu合金纳米颗粒(PdCu-MoOx),以促进氢和羟基在非均相界面上的溢出。无定形MoOx簇中多样的氧缺陷与Mo优异的亲氧性协同作用,促进OH*的吸附和迁移。铜的引入优化了MoOx簇与合金界面处能带的匹配,减弱了界面电荷的积累,降低了合金表面的HBE,从而降低了中间OH*和H*转移的能垒。WO3颜色变化实验和覆盖CO剥离伏安图的结果分别揭示了H*和OH*在界面处的迁移。动力学计算表明,PdCu-MoOx的氢溢出最后一步的动能势垒仅为0.08 eV,远低于MoOx簇修饰Pd (Pd-MoOx)的0.51 eV。PdCu-MoOx在低过电位为50 mV时表现出较高的HOR动力学活性(238.1 mA mgPd-1),是最佳的pd基催化剂之一,使用PdCu-MoOx阳极的阴离子交换膜燃料电池(aemfc)的阳极质量标准化峰值功率密度高达9.96 W mgPGM-1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
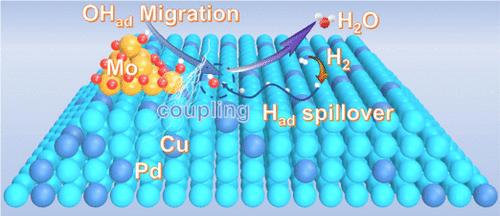
Boosting Hydroxyl Migration over Palladium-Based Catalysts to Enhance the Alkaline Hydrogen Oxidation
Pd-based materials, as typical hydrogen storage materials, usually have a strong hydrogen binding energy (HBE) and a poor hydroxyl binding energy (OHBE), which hinders the transfer of intermediates and results in a poor hydrogen oxidation reaction (HOR) activity at low potentials. Here, MoOx cluster-modified PdCu alloy nanoparticles (PdCu-MoOx) were prepared to promote hydrogen and hydroxyl spillover at the heterogeneous interface. The diverse oxygen defects in the amorphous MoOx clusters synergize with the excellent oxophilicity of Mo to boost the adsorption and migration of OH*. The introduction of copper optimizes the matching of the energy band at the interface between MoOx clusters and the alloy, weakening the accumulation of interfacial charges and reducing the HBE on the alloy surface, which results in a lower energy barrier for intermediate OH* and H* transfer. The results of the WO3 color change experiments and overlaid CO stripping voltammograms revealed the migration of H* and OH* at the interface, respectively. Kinetic calculations indicate a kinetic energy barrier of only 0.08 eV for the last step of hydrogen overflow at PdCu-MoOx, much lower than the 0.51 eV for MoOx cluster-modified Pd (Pd-MoOx). PdCu-MoOx exhibited high HOR kinetic activity (238.1 mA mgPd–1) at a low overpotential of 50 mV, thereby making it one of the best Pd-based catalysts, and the anion exchange membrane fuel cells (AEMFCs) with a PdCu-MoOx anode delivers a high anode mass-normalized peak power density of 9.96 W mgPGM–1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


