梯度机电微环境的耦合通过Ca2 +信号激活促进界面再生
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
天然肌腱-骨界面通过胶原纤维的梯度排列和矿化实现应力分散;然而,手术修复往往导致应力集中和再生失败。为了解决这个问题,研究人员开发了一种可植入的、具有磁响应的梯度压电水凝胶系统,该系统通过使用静态磁场对齐短纤维(cmsf)来模拟自然界面,从而为细胞分化创造了一个动态的机械微环境。在动态磁场作用下,梯度机械振动通过细胞力学感知增强细胞分化,而压电信号进一步促进组织再生。基于gelma的水凝胶通过静电纺丝和磁调节将CMSFs和BaTiO3纳米颗粒整合在一起,从而通过Calcr途径维持干细胞稳态,并激活Ca2 +信号来驱动多系分化。在大鼠跟腱-骨损伤模型中,该系统成功再生了连续的四层组织结构,恢复了80%的原始拉伸强度,在组织学上优于对照组。本研究开创了1)植入物的梯度机械编程和2)“磁力-力-电”多物理场的协同界面再生,为复杂组织修复提供了一种创新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
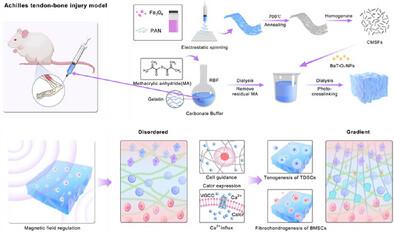
The Coupling of Gradient Mechano-Electrical Microenvironments Promotes Interface Regeneration via Ca2⁺ Signaling Activation
The natural tendon-bone interface achieves stress dispersion through its gradient arrangement of collagen fibers and mineralization; however, surgical repair often leads to stress concentration and regeneration failure. To address this issue, an implantable, magnetically responsive gradient piezoelectric hydrogel system is developed that mimics the natural interface by aligning short fibers (CMSFs) using a static magnetic field, thereby creating a dynamic mechanical microenvironment for cell differentiation. Under a dynamic magnetic field, gradient mechanical vibrations enhance differentiation through cellular mechanosensing, while piezoelectric signals further promote tissue regeneration. The GelMA-based hydrogel integrates CMSFs and BaTiO3 nanoparticles through electrospinning and magnetic regulation, thereby maintaining stem cell homeostasis via the Calcr pathway and activating Ca2⁺ signaling to drive multilineage differentiation. In the rat Achilles tendon-bone injury model, the system successfully regenerates a continuous four-layer tissue structure, restoring 80% of the native tensile strength and outperforming control groups histologically. This study pioneers 1) gradient mechanical programming in implants and 2) the synergy of “magnetic-force-electric” multi-physical fields for interface regeneration, offering an innovative strategy for complex tissue repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


