ai驱动的纳米士兵在感染治疗中精确破坏生物膜的细胞外防御
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
细菌生物膜感染目前是一个全球性的健康挑战,因为生物膜的存在阻止了药物的渗透并引发了难以根除的炎症。开发新的策略来治疗由细菌膜引起的慢性炎症是至关重要的。在本研究中,设计、合成并自组装成纳米粒子(TDTNI NPs),并命名为TDTNI聚集致发射发光团(AIEgens)。它们具有高效的光动力治疗(PDT)和光热治疗(PTT)特性,能有效灭活金黄色葡萄球菌(S. aureus)和牙龈卟啉单胞菌(P. gingivalis),抑制生物膜细胞外聚合物质(EPS)的形成,减少细菌粘附。金黄色葡萄球菌感染小鼠伤口实验和大鼠牙周炎治疗实验进一步证明,TDTNI NPs具有良好的细菌膜清除性能和eps靶向能力,促进伤口愈合和牙周炎治疗,并且对生物体本身几乎没有副作用。此外,它们可以去除附着在牙釉质表面的污渍,而不会损伤牙釉质和牙周组织。它们具有很强的光声/光热成像能力,并且在体内实验证明了出色的分辨率和组织穿透能力。鉴于示范示范,TDTNI NPs在治疗细菌生物膜感染伤口和牙周炎方面的优势使其成为治疗细菌生物膜感染的有希望的候选者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
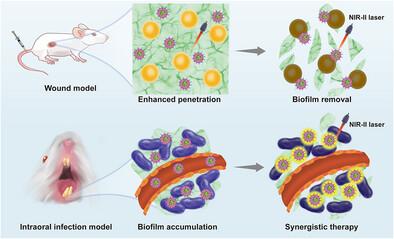
AIE-Powered Nanosoldiers for Precision Disruption of Biofilm's Extracellular Defense in Infection Treatment
Bacterial biofilm infections are currently a global health challenge because the presence of biofilms prevents drug penetration and triggers inflammation that is difficult to eradicate. The need to develop new strategies to treat chronic inflammation caused by bacterial films is crucial. In this study, aggregation-induced emission luminophores (AIEgens) named TDTNI are designed, synthesized, and self-assembled into nanoparticles (TDTNI NPs). They have highly efficient photodynamic therapy (PDT) and photothermal therapy (PTT) properties, effectively inactivating Staphylococcus aureus (S. aureus) and Porphyromonas gingivalis (P. gingivalis), inhibiting the formation of extracellular polymeric substances (EPS) in biofilms, and reducing bacterial adhesion. S. aureus-infected mouse wound experiments and rat periodontitis treatment experiments further demonstrated that TDTNI NPs have good bacterial membrane-scavenging properties and EPS-targeting ability, promote wound healing and periodontitis treatment, and have almost no side effects on the organisms themselves. Furthermore, they can eliminate stains that adhere to the enamel surface without damaging the enamel and periodontal tissues. They have strong photoacoustic/photothermal imaging capabilities, and in vivo experiments have demonstrated excellent resolution and tissue penetration capabilities. Given the exemplary demonstration, the superiority of TDTNI NPs in treating bacterial biofilm-infected wounds and periodontitis makes them promising candidates for application in the treatment of bacterial biofilm infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


