多模态阳离子交换型酪氨酸手性固定相的合成及其在高效液相色谱中的应用
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
使用手性固定相(CSPs)的高效液相色谱(HPLC)是分离对映体最常用的技术之一。在最广泛的csp中,那些含有离子交换器作为手性选择器(SOs)的csp已成为极性和可极化化合物分离的有力工具。除了成熟的商业材料,如金鸡纳生物碱基手性弱阴离子交换剂(WAX)和两性离子离子交换相(ZWIX),手性阳离子交换csp是一个有价值的选择。这些材料已被证明广泛适用于外消旋胺的对映体分离,包括广泛的药物化合物。本文介绍了新型多模态手性阳离子交换剂的设计、合成和色谱评价。这些CSP创新性地结合了供体-受体相互作用和阳离子交换相互作用的原理,通过实现一个3,5-二硝基苯甲酰酪氨酸核心,以磺酸(即强阳离子交换剂,scx型,CSP I和II)或羧酸部分(即弱阳离子交换剂,wcx型,CSP III)作为各自的离子交换位点。该合成策略的一个关键特征是通过铜(I)催化叠氮化物-炔环加成反应(即点击化学)将手性选择剂有效地共价固定在二氧化硅载体上。在极性有机(PO)模式条件下,系统地研究了csp对各种手性碱分析物的对映体分离性能。研究了流动相组成(包括溶剂极性、酸性和碱性添加剂的性质和浓度)对保留以及化学和对映体选择性的影响。此外,在正常相(NP)条件下,可以证明不带电分析物的成功对映体分离。这项工作介绍了改进的基于酪氨酸的csp,有效地整合了供体-受体和阳离子交换功能,使它们成为具有挑战性的手性分离可用工具包的多功能和强大的补充。本文章由计算机程序翻译,如有差异,请以英文原文为准。
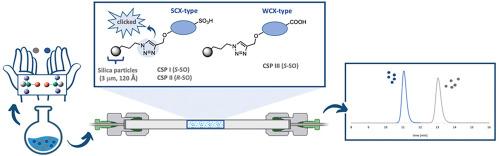
Multimodal cation exchange-type tyrosine-based chiral stationary phases: Synthesis and applications in high-performance liquid chromatography
Background
High-performance liquid chromatography (HPLC) using chiral stationary phases (CSPs) is among the most prevalent techniques for the separation of enantiomers. Among the most widespread CSPs, those containing ion-exchangers as chiral selectors (SOs) have emerged as powerful tools for the separation of polar and polarizable compounds. In addition to well-established commercial materials, such as Cinchona alkaloid-based chiral weak anion exchangers (WAX) and zwitterionic ion-exchange phases (ZWIX), chiral cation-exchange CSPs represent a valuable alternative. These materials have demonstrated broad applicability in the enantioseparation of racemic amines, encompassing a wide spectrum of pharmaceutical compounds. This study presents the design, synthesis, and chromatographic evaluation of novel multimodal chiral cation exchangers for HPLC.Results
These CSPs innovatively combine the principles of both donor-acceptor and cation-exchange interactions by implementing a 3,5-dinitrobenzoyl tyrosine core with either a sulfonic acid (i.e., strong cation exchanger, SCX-type, CSP I and II) or a carboxylic acid moiety (i.e., weak cation exchanger, WCX-type, CSP III) as the respective ion exchange sites. A key feature of the synthetic strategy was the efficient covalent immobilization of the chiral selectors onto the silica support via a copper(I)-catalyzed azide-alkyne cycloaddition reaction (i.e., click chemistry). The chromatographic performance of the CSPs was systematically investigated under polar organic (PO) mode conditions for the enantioseparation of various chiral basic analytes. The influence of the mobile phase composition – including solvent polarity and the nature and concentration of acidic and basic additives – on retention as well as chemo- and enantioselectivity was thoroughly studied. Furthermore, the successful enantioseparation of uncharged analytes under normal phase (NP) conditions could be demonstrated.Significance
This work introduces improved tyrosine-based CSPs that effectively integrate donor-acceptor and cation-exchange functionalities, rendering them a versatile and powerful addition to the available toolkit for challenging chiral separations.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


