耐腐蚀海水分解阳极Co2(OH)3Cl和Ni2(OH)3Cl的稳定性、电子性质和协同光电催化活性:基于连续介质溶剂化模型的恒电位动力学
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
虽然光电催化在淡水分裂中表现出色,但海水环境带来了严峻的挑战(阳极腐蚀和竞争反应)。本文首次采用连续溶剂化模型,基于等势动力学研究了海水裂解催化剂Co2(OH)3Cl和Ni2(OH)3Cl的协同光电催化作用。结果表明:(1)两种化合物均为直接带隙半导体,具有紫外-可见光吸收特性,适于水分解;(2)导带最小值(CBM)的热力学取向使自发析氢反应(HER)成为可能,而析氧反应(OER)的激活需要阳极偏置(Co2(OH)3Cl/Ni2(OH)3Cl为1.12 V/0.84 V);(3) OER过电位(Ni2(OH)3Cl 0.48 V < Co2(OH)3Cl 0.76 V)均低于析氯反应(CER)过电位(1.62 V/1.82 V),抑制了副反应,增强了耐蚀性。实际稳定性计算采用多方面的方法:热力学(负内聚能:−0.631/−0.376 eV/原子),机械,动态和热稳定性。我们的发现促进了海水分裂的协同光电催化的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
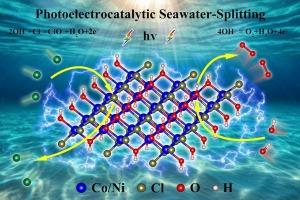
Stability, electronic properties, and synergistic photoelectrocatalytic activity in corrosion-resistant seawater-splitting anodes Co2(OH)3Cl and Ni2(OH)3Cl: constant-potential dynamics via continuum solvation models
While photoelectrocatalysis excels in freshwater splitting, seawater environments introduce severe challenges (anode corrosion and competing reactions). In this paper, we investigated synergistic photoelectrocatalysis of seawater splitting catalysts Co2(OH)3Cl and Ni2(OH)3Cl based on the constant-potential dynamics via continuum solvation models for the first time. Results indicated that: (1) Both compounds are direct band gap semiconductors with UV–Vis light absorption suitable for water splitting.; (2) Thermodynamic alignment of the conduction band minimum (CBM) enables spontaneous hydrogen evolution reaction (HER), while the oxygen evolution reaction (OER) activation necessitates anodic biases (1.12 V/0.84 V for Co2(OH)3Cl/Ni2(OH)3Cl); (3) OER overpotential (Ni2(OH)3Cl 0.48 V < Co2(OH)3Cl 0.76 V) are both lower than the chlorine evolution reaction (CER) overpotential (1.62 V/1.82 V), suppressing side reactions and enhancing corrosion resistance. Practical stability calculations employed multifaceted approaches: thermodynamic (negative cohesive energy: −0.631/−0.376 eV/atom), mechanical, dynamic, and thermal stability. Our findings facilitate the development of synergistic photoelectrocatalysis for seawater splitting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


