超声加速Ti3FeBi5O15纳米片放大活性氧风暴/铁下沉触发抗肿瘤免疫治疗
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
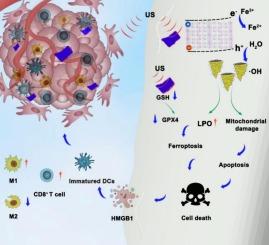
细胞内Fe2+离子可用性不足和肿瘤微环境缺氧严重阻碍了铁下垂的效率。目前迫切需要在铁下垂诱导剂的开发和递送以及免疫微环境的重编程方面进行突破性的研究。本文设计了Ti3FeBi5O15 (TFBO)压电纳米片,通过超声(US)激活来放大铁凋亡并触发免疫原性细胞死亡。TFBO纳米片具有窄带隙(2.03 eV)和高压电系数(d33 = 22.1 pm V−1),能够在US辐照下实现高效的电子空穴分离。值得注意的是,TFBO纳米片产生了一个内部压电场,在US照射下促进了Fe3+到Fe2+的转化和H2O的生成,同时在US照射下消耗细胞内GSH,导致脂质过氧化和GPX4抑制。重要的是,TFBO纳米片激活免疫原性细胞死亡(ICD),刺激抗肿瘤免疫反应。因此,结合其高生物相容性,有效的肿瘤抑制性能和最小的全身毒性,TFBO代表了一种机制独特且翻译前景广阔的肿瘤治疗铁下垂放大器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ultrasound-accelerated Ti3FeBi5O15 nanosheets amplify reactive oxygen species storms/ferroptosis to trigger antitumor immunotherapy
Insufficient intracellular Fe2+ ions availability and hypoxic tumor microenvironment significantly hamper the efficiency of ferroptosis. There is an urgent need for breakthrough research in the development and delivery of ferroptosis inducers and the reprogramming of the immune microenvironment. Herein, Ti3FeBi5O15 (TFBO) piezoelectric nanosheets were engineered to amplify ferroptosis and trigger immunogenic cell death via ultrasound (US) activation. TFBO nanosheets feature a narrow bandgap (2.03 eV) and high piezoelectric coefficient (d33 = 22.1 pm V−1), enabling efficient electron-hole separation under US irradiation. Notably, TFBO nanosheets generate an internal piezoelectric field that promotes Fe3+ to Fe2+ conversion and ·OH generation from H2O under US irradiation, while simultaneously depleting intracellular GSH under US irradiation, leading to lipid peroxidation and GPX4 suppression. Importantly, TFBO nanosheets activate immunogenic cell death (ICD), stimulating antitumor immune responses. Therefore, combined with its high biocompatibility, effective tumor inhibiting performance, and minimal systemic toxicity, TFBO represents a mechanistically distinct and translationally promising ferroptosis amplifier for tumor therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


