单细胞沿受限触致梯度移动
IF 18.4
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
摘要
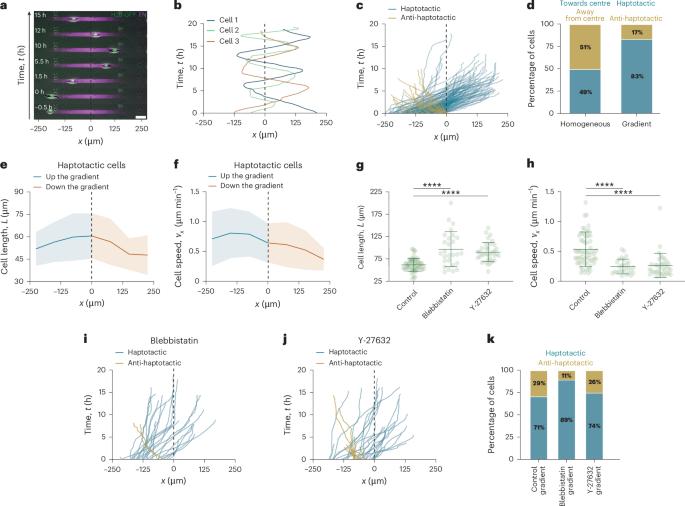
趋向性是细胞沿着细胞外基质密度梯度定向迁移的过程,是形态发生、免疫反应和癌症侵袭的核心。一般认为,细胞对这些梯度的反应是向配体密度最高的区域定向迁移。与这一观点相反,这里我们表明,暴露于微图案纤维连接蛋白梯度的细胞表现出广泛的复杂轨迹,包括定向的触觉迁移梯度,但也有线性振荡和长时间迁移梯度的圆圈。为了解释这种行为,我们建立了一个基于粗粒度分子离合器模型的触致细胞迁移的生物物理模型,该模型与持续的随机极性动力学相结合。虽然最初的触致迁移可以用细胞前后的差异摩擦来解释,但在更长的时间尺度上观察到的复杂轨迹是由差异摩擦、持久性和物理限制之间的相互作用产生的。总的来说,我们的研究表明,限制和持续调节细胞感知和响应触觉线索的能力,并为理解细胞如何在复杂环境中导航提供了一个框架。趋向性传统上被认为是蛋白质梯度上的迁移。现在表明,细胞也可以沿梯度振荡或迁移。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Single-cell migration along and against confined haptotactic gradients
Haptotaxis is the process of directed cell migration along gradients of extracellular matrix density and is central to morphogenesis, immune responses and cancer invasion. It is commonly assumed that cells respond to these gradients by migrating directionally towards the regions of highest ligand density. In contrast with this view, here we show that cells exposed to micropatterned fibronectin gradients exhibit a wide range of complex trajectories, including directed haptotactic migration up the gradient but also linear oscillations and circles with extended periods of migration down the gradient. To explain this behaviour, we developed a biophysical model of haptotactic cell migration based on a coarse-grained molecular clutch model coupled to persistent stochastic polarity dynamics. Although initial haptotactic migration is explained by the differential friction at the front and back of the cell, the observed complex trajectories over longer timescales arise from the interplay between differential friction, persistence and physical confinement. Overall, our study reveals that confinement and persistence modulate the ability of cells to sense and respond to haptotactic cues and provides a framework for understanding how cells navigate complex environments. Haptotaxis was traditionally seen as migration up protein gradients. It is now shown that cells can also oscillate or migrate down the gradient.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


