硝酸镭、钡和铅在CH3COOH-HNO3-H2O体系中的溶解度
IF 1
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
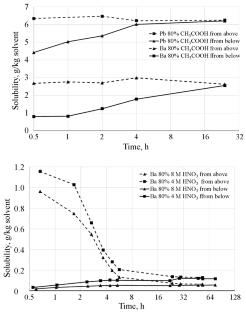
测定了硝酸镭、硝酸钡和硝酸铅在醋酸及其与0.5 ~ 16 M硝酸混合水溶液中的溶解度。在乙酸水溶液中,镭、钡和硝酸铅的溶解度随着CH3COOH质量分数的增加而单调降低,在冰醋酸中,它们的溶解度分别为0.0027、0.010和0.069 g/kg溶剂。对于醋酸和硝酸的混合物,溶解度通过相应的96-99 wt % CH3COOH的最小值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Solubility of Radium, Barium, and Lead Nitrates in the CH3COOH–HNO3–H2O System
The solubility of radium, barium, and lead nitrates in aqueous solutions of acetic acid and its mixtures with 0.5 to 16 M nitric acid was determined. For aqueous solutions of acetic acid, the solubilities of radium, barium, and lead nitrates decrease monotonically with increasing mass fraction of CH3COOH, and for glacial acetic acid they are 0.0027, 0.010, and 0.069 g/kg solvent, respectively. For mixtures of acetic and nitric acids, the solubilities pass through a minimum corresponding to 96–99 wt % CH3COOH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Radiochemistry
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
1.30
自引率
33.30%
发文量
51
期刊介绍:
Radiochemistry is a journal that covers the theoretical and applied aspects of radiochemistry, including basic nuclear physical properties of radionuclides; chemistry of radioactive elements and their compounds; the occurrence and behavior of natural and artificial radionuclides in the environment; nuclear fuel cycle; radiochemical analysis methods and devices; production and isolation of radionuclides, synthesis of labeled compounds, new applications of radioactive tracers; radiochemical aspects of nuclear medicine; radiation chemistry and after-effects of nuclear transformations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


