基于99mtc标记的黑色素瘤SPECT/CT成像的CXCR4环肽分子对接创新设计
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
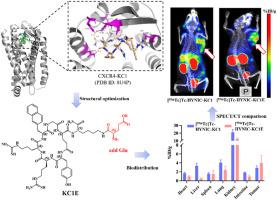
趋化因子受体CXCR4与肿瘤的生长、转移、侵袭和预后密切相关,是肿瘤诊断和治疗的重要靶点。结果成功合成了环状肽KC1和KC1E及其放射性标记偶联物[99mTc] tc - hyic -KC1和[99mTc] tc - hyic -KC1E。利用表面等离子体共振(SPR)技术评估KC1对CXCR4受体的结合亲和力。此外,系统地评估了放射性示踪剂的主要理化和生物学特性,包括油水分配系数(LogP)、生理盐水溶液和小鼠血清中的体外稳定性、B16-F10肿瘤小鼠模型的体内SPECT/CT成像性能以及静脉给药后的生物分布特征。研究人员设计了一种新的环状肽KC1,并证明其可选择性结合CXCR4受体。通过分子对接模拟来阐明KC1和CXCR4之间潜在的相互作用机制。合成了放射性标记衍生物[99mTc]Tc-HYNIC-KC1E,并在B16-F10肿瘤小鼠中显示出良好的体内成像性能,支持其作为cxcr4阳性肿瘤显像剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Innovative Design of CXCR4 Cyclic Peptide via Molecular Docking for 99mTc-Labeled SPECT/CT Imaging in Melanoma
Backgroud
The chemokine receptor CXCR4 is a crucial target for the diagnosis and treatment of cancer due to its close association with tumor growth, metastasis, invasiveness, and prognosis.Results
The cyclic peptides KC1 and KC1E, along with their radiolabeled conjugates [99mTc]Tc-HYNIC-KC1 and [99mTc]Tc-HYNIC-KC1E, were successfully synthesized. The binding affinity of KC1 toward the CXCR4 receptor was evaluated using Surface Plasmon Resonance (SPR). Furthermore, key physicochemical and biological properties of the radiotracers were systematically assessed, including the oil-water partition coefficient (LogP), in vitro stability in normal saline solution and mouse serum, in vivo SPECT/CT imaging performance in a B16-F10 tumor-bearing mouse model, and biodistribution profiles following intravenous administration.Significance
A novel cyclic peptide, KC1, was designed and shown to exhibit selective binding to the CXCR4 receptor. Molecular docking simulations were performed to elucidate the potential interaction mechanism between KC1 and CXCR4. The radiolabeled derivative [99mTc]Tc-HYNIC-KC1E was synthesized and demonstrated favorable in vivo imaging performance in B16-F10 tumor-bearing mice, supporting its potential as a promising imaging agent for CXCR4-positive tumors.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


