含钾石墨在Co3O4上诱导的界面工程用于甘油选择性电氧化制备乙醇酸盐
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
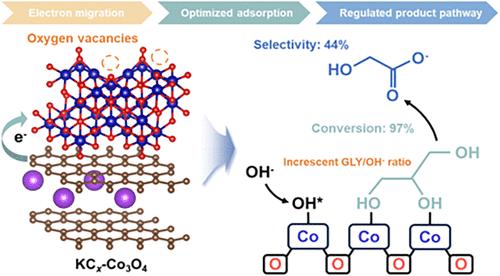
通过电催化将甘油转化为乙醇酸提供了一种使剩余甘油增值和缓解能源困境的策略。然而,如何精确控制羟基官能团的氧化和C-C键的断裂仍然是一个挑战。在此,我们开发了一种界面工程策略,利用钾石墨嵌入化合物(K-GIC)来调节Co3O4,显著提高了电催化甘油氧化反应(GOR)生产乙醇酸盐的性能。具体来说,从富电子的KCx到Co3O4的界面电荷转移增加了Co和O原子的电子密度。同时,由于电子效应和晶格畸变,Co3O4上产生了更多的氧空位。结果表明,在催化界面上,甘油和活性氧OH -的吸附能力显著增强,吸附比明显调节。因此,在表面催化活性增强的同时,甘油的深度氧化和二次C-C解理作用减弱,有利于乙醇酸的生成。原位衰减全反射表面增强红外吸收光谱(ATR-SEIRAS)和密度泛函理论(DFT)计算进一步揭示了乙醇酸途径中能量势垒的降低。KCx-Co3O4具有较高的乙醇酸选择性和法拉第效率,分别达到44%和37%,甘油转化率为97%。本研究为界面工程设计增值甘油电氧化催化剂提供了实际指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interface Engineering Induced by Potassium-Intercalated Graphite on Co3O4 for Selective Electrooxidation of Glycerol into Glycolate
Transforming glycerol into glycolic acid through electrocatalysis offers a strategy for valorizing surplus glycerol and alleviating the energy dilemma. However, it remains challenging to precisely control the oxidation of the hydroxyl functional group and the fracture of the C–C bond. Herein, we develop an interface engineering strategy utilizing potassium graphite intercalation compounds (K-GIC) to modulate Co3O4, achieving significantly enhanced performance in producing glycolate from the electrocatalytic glycerol oxidation reaction (GOR). Specifically, the interfacial charge transfer from electron-rich KCx to Co3O4 increases the electronic density of Co and O atoms. Meanwhile, more oxygen vacancies were generated on Co3O4 due to the electronic effect and lattice distortion. Consequently, the markedly enhanced adsorption capacities and modulated adsorption ratio of glycerol and reactive oxygen species OH– are determined on the catalytic interface. As a result, while the surface catalytic activity is enhanced, the deep oxidation of glycerol and secondary C–C cleavage are weakened, which is beneficial for the generation of glycolate. In situ attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) and density functional theory (DFT) calculations further unlock the decrease in the energy barrier in the pathway toward glycolate. The KCx-Co3O4 exhibits high glycolate selectivity and faradaic efficiency, reaching 44% and 37%, respectively, with a glycerol conversion of 97%. This work provides practical guidance for interface engineering in designing catalysts for value-added glycerol electrooxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


