含铁(II)矿物在氧化环境中形成活性氧化剂的研究进展
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
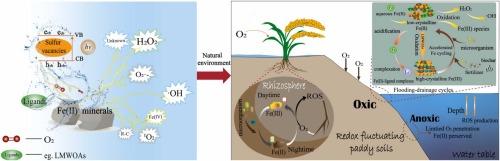
铁是地壳中含量第四丰富的元素。它在调节活性氧(ROS)的形成和其他地球化学过程中起着关键作用。系统地综述了氧化条件下活性氧化剂的产生机理、影响因素及其在环境中的存在。在含氧水生环境中,含铁(II)的矿物质可以驱动活性氧的产生,包括ROS、四价铁(Fe(IV))、碳中心自由基(R-C•)和其他未表征的活性物质。关键的形成过程包括铁(II)矿物介导的氧还原的单电子还原和双电子还原、太阳照射、微生物活性、硫缺陷介导的三条途径(铁(III)与H2O反应生成•OH、铁(II)/O2与硫中间体反应生成H2O2)以及铁(II)-草酸氧化过程中形成的碳中心自由基。此外,pH≥7条件下Fe(IV)的生成和金属离子介导的1O2的生成也起着重要作用。环境因素、铁(II)矿物形成(如;本文综述了共同调节氧化剂产率的配体化学、结晶度、表面缺陷等。来自河岸带、湖泊沉积物、海岸系统和水稻土的实地证据表明,活性氧在污染物降解和微生物代谢调节中的作用。指出了在自然条件下非羟基自由基种类、协同作用、反应途径和界面反应动力学的原位量化方面存在的重大空白,并对未来的研究提出了建议。为富铁环境中涉及活性氧化剂的污染控制和环境修复提供了全面的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Formation of reactive oxidants from Fe(II)-bearing minerals in oxic environments: A review
Iron is the fourth most abundant element in the earth’s crust. It plays a pivotal role in regulating reactive oxidants including reactive oxygen species (ROS) formation and other geochemical processes. The generation mechanisms of reactive oxidants, the influencing factors and their environmental occurrence under oxic conditions are systematically reviewed. In oxic aquatic environments, Fe(II)-containing minerals can drive the production of reactive oxidants, including ROS, tetravalent iron (Fe(IV)), carbon-centered free radicals (R-C•) and other uncharacterized reactive species. The key formation processes include one-electron reduction and two-electron reduction of oxygen reduction mediated by Fe(II) minerals, solar irradiation, microbial activity, three sulfur defect-mediated pathways (production of •OH from reaction between  Fe(III) and H2O, and reaction of Fe(II)/O2 with sulfur intermediates, as well as formation of H2O2 in the reaction between
Fe(III) and H2O, and reaction of Fe(II)/O2 with sulfur intermediates, as well as formation of H2O2 in the reaction between  S(-II) and O2) and carbon-centered radicals formed during Fe(II)-oxalate oxidation. Furthermore, Fe(IV) formation at pH ≥ 7 and metal ion-mediated 1O2 generation play significant roles. The environmental factors, Fe(II) minerals speciation (eg., crystallinity, surface defects) and ligand chemistry that together modulate oxidant yields are reviewed. Field evidence is compiled from riparian zones, lake sediments, coastal systems, and paddy soils, demonstrating ROS roles in pollutant degradation and microbial metabolism regulation. Significant gaps in in-situ quantifying the contributions of non-hydroxyl radical species, synergies, reaction pathways and interfacial reaction dynamics under natural conditions are proposed with suggestions for future research. It can provide a comprehensive understanding for pollution control and environmental remediation involving reactive oxidants in Fe(II)-rich environments.
S(-II) and O2) and carbon-centered radicals formed during Fe(II)-oxalate oxidation. Furthermore, Fe(IV) formation at pH ≥ 7 and metal ion-mediated 1O2 generation play significant roles. The environmental factors, Fe(II) minerals speciation (eg., crystallinity, surface defects) and ligand chemistry that together modulate oxidant yields are reviewed. Field evidence is compiled from riparian zones, lake sediments, coastal systems, and paddy soils, demonstrating ROS roles in pollutant degradation and microbial metabolism regulation. Significant gaps in in-situ quantifying the contributions of non-hydroxyl radical species, synergies, reaction pathways and interfacial reaction dynamics under natural conditions are proposed with suggestions for future research. It can provide a comprehensive understanding for pollution control and environmental remediation involving reactive oxidants in Fe(II)-rich environments.
 Fe(III) and H2O, and reaction of Fe(II)/O2 with sulfur intermediates, as well as formation of H2O2 in the reaction between
Fe(III) and H2O, and reaction of Fe(II)/O2 with sulfur intermediates, as well as formation of H2O2 in the reaction between  S(-II) and O2) and carbon-centered radicals formed during Fe(II)-oxalate oxidation. Furthermore, Fe(IV) formation at pH ≥ 7 and metal ion-mediated 1O2 generation play significant roles. The environmental factors, Fe(II) minerals speciation (eg., crystallinity, surface defects) and ligand chemistry that together modulate oxidant yields are reviewed. Field evidence is compiled from riparian zones, lake sediments, coastal systems, and paddy soils, demonstrating ROS roles in pollutant degradation and microbial metabolism regulation. Significant gaps in in-situ quantifying the contributions of non-hydroxyl radical species, synergies, reaction pathways and interfacial reaction dynamics under natural conditions are proposed with suggestions for future research. It can provide a comprehensive understanding for pollution control and environmental remediation involving reactive oxidants in Fe(II)-rich environments.
S(-II) and O2) and carbon-centered radicals formed during Fe(II)-oxalate oxidation. Furthermore, Fe(IV) formation at pH ≥ 7 and metal ion-mediated 1O2 generation play significant roles. The environmental factors, Fe(II) minerals speciation (eg., crystallinity, surface defects) and ligand chemistry that together modulate oxidant yields are reviewed. Field evidence is compiled from riparian zones, lake sediments, coastal systems, and paddy soils, demonstrating ROS roles in pollutant degradation and microbial metabolism regulation. Significant gaps in in-situ quantifying the contributions of non-hydroxyl radical species, synergies, reaction pathways and interfacial reaction dynamics under natural conditions are proposed with suggestions for future research. It can provide a comprehensive understanding for pollution control and environmental remediation involving reactive oxidants in Fe(II)-rich environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


