自旋极化电子输运促进氧还原反应
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
析氧反应(OER)和氧还原反应(ORR)是电解和燃料电池效率的核心,涉及氧的顺磁三重态基态和水的单线态基态。在这里,我们证明了自旋极化电流增强了ORR活动。在钕(Nd)磁体上使用镀银镍电极,我们观察到当银层比银的自旋扩散长度更薄时,ORR性能达到最大,在这种情况下,电极-电解质界面的自旋排列保持不变。相反,较厚银层的实验导致自旋松弛和电催化活性降低。该系统的模型描述表明,界面处存在大量的自旋极化,同时存在较大的双电子转移,满足ORR过程中的角动量守恒。这些发现突出了自旋选择性电荷转移的关键作用,并为氧电催化反应途径的控制提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
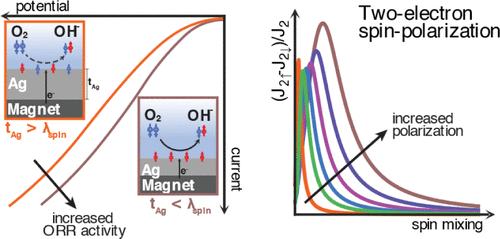
Spin-Polarized Electron Transport Promotes the Oxygen Reduction Reaction
Oxygen evolution (OER) and oxygen reduction (ORR) reactions are central to the efficiency of electrolysis and fuel cells, involving the paramagnetic triplet ground state of oxygen and the singlet ground state of water. Here, we demonstrate that spin-polarized currents enhance the ORR activity. Using a silver-coated nickel electrode over a neodymium (Nd) magnet, we observed that ORR performance is maximized when the Ag layer is thinner than the spin diffusion length of silver─conditions under which spin alignment at the electrode–electrolyte interface is maintained. In contrast, experiments with thicker Ag layers lead to spin relaxation and diminished electrocatalytic activity. A model description of this system shows that a substantial spin polarization at the interface is accompanied by a large two-electron transfer, which satisfies conservation of angular momentum during ORR. These findings highlight the critical role of spin-selective charge transfer and offer insights into the control of reaction pathways in oxygen electrocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


