尿素电合成中的混价金属间化合物
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
高效的尿素电合成依赖于精确控制两个平行还原反应的动力学,即二氧化碳(CO2)还原和硝酸盐(NO3 -)还原。然而,一个主要的挑战在于在原子尺度上构建稳定的和不同的活性位点,这对于同步这两个反应的反应动力学和促进C-N耦合至关重要。在此,我们引入了一种混合价金属间化合物Cu2Sb,以Cu+ - cu2 +双位点为模块构建块来调节和同步CO2和NO3 -还原动力学。机理研究表明,所构建的双位点稳定了*CO和*NO中间体,降低了C-N偶联的能垒,显著提高了尿素合成效率。在−0.4 V下,Cu2Sb催化剂的尿素产率为22.9 mmol h - 1 gcat-1,法拉第效率为64.9%,在200 h内保持稳定,超过了之前报道的大多数催化剂。这项工作开创了Mv-IMCs中多价活性位点的精确构建,为设计适合增值有机合成的高性能电催化剂建立了范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
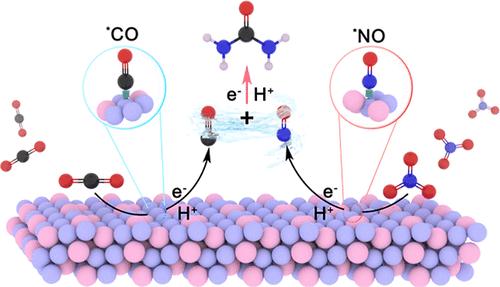
Mixed-Valence Intermetallic Compounds for Urea Electrosynthesis
Efficient urea electrosynthesis relies on precisely controlling the kinetics of two parallel reduction reactions, i.e., carbon dioxide (CO2) reduction and nitrate (NO3–) reduction. However, a major challenge lies in constructing stable and different active sites at the atomic scale, which are essential for synchronizing the reaction kinetics of these two reactions and facilitating C–N coupling. Herein, we introduce a mixed-valence intermetallic compound (Mv-IMC) Cu2Sb, featuring Cu+–Cu2+ dual-sites as modular building blocks to regulate and synchronize CO2 and NO3– reduction kinetics. Mechanistic studies reveal that the constructed dual-sites stabilize *CO and *NO intermediates, lower the energy barrier of C–N coupling, and significantly enhance the urea synthesis efficiency. The Cu2Sb catalyst achieves a urea yield of 22.9 mmol h–1 gcat–1 with a Faradaic efficiency of 64.9% at −0.4 V, maintaining stability over 200 h, surpassing most previously reported catalysts. This work pioneers the precise construction of multivalent active sites in Mv-IMCs, establishing a paradigm for designing high-performance electrocatalysts tailored to value-added organic synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


