微泡阵列微生理系统的设计、制造与表征
IF 5.4
2区 工程技术
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
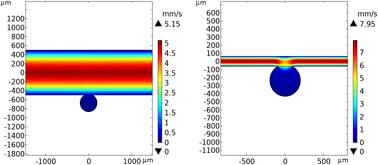
微泡(MB)技术非常适合集成到微生理系统(MPS)中,用于高通量三维(3D)组织培养、药物筛选和毒性测试。mb是球形隔室,以阵列形式生产纳升体积。在这里,我们提出了一种新的混合mb流体MPS,将3D组织培养与控制流体流动相结合,以改善营养输送,废物清除和生理相关性。计算流体动力学(CFD)模拟了速度和溶质扩散曲线作为MB宽高比(AR)的函数,并通过荧光聚苯乙烯微球光学跟踪进行了验证。模拟结果显示,mb内流速比主通道低200倍以上,具有明显的速度解耦和剪切阻尼效应,从而在保持低剪切微环境的同时,实现了高流速的高效交换,这是组织培养的最佳选择。此外,在高流量条件下,在单个mb中空间区隔的组织不会移位。考虑到高通道流速,与MB微环境解耦,可以使用比微流控装置更容易制造和控制的微流控装置。模拟还表明,AR值在2和3之间的MBs提供了营养转运和细胞分泌因子保留之间的平衡。相比之下,直井在AR >; 2处出现了流动分裂和乳酸积聚,这突出了球形MB几何结构的一个关键优势。我们使用熔融沉积建模(FDM) 3D打印和一种新的成型策略制造了一种微流体MB装置,以创建光学清晰,无泄漏的流动通道。在该装置下流动培养的小鼠唾液腺组织显示保留了腺泡细胞标记基因表达,减少了导管标记,支持了动态流动增强组织保真度的假设。这种mb流体平台支持可扩展的高含量3D培养系统,适用于药物发现和毒理学中的类器官,肿瘤球体和组织模拟应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, fabrication, and characterization of a microbubble array microphysiological system
Microbubble (MB) technology is uniquely suited for integration into microphysiological systems (MPS) for high throughput three-dimensional (3D) tissue culture, drug screening and toxicity testing. MBs are spherical compartments with nanoliter volumes produced in an array format. Here, we present a novel hybrid MB-fluidic MPS that combines 3D tissue culture with controlled fluid flow to improve nutrient delivery, waste removal, and physiological relevance. Computational fluid dynamics (CFD) simulations were used to model velocity and solute diffusion profiles as a function of MB aspect ratio (AR), with validation by fluorescent polystyrene microsphere optical tracking. Simulations reveal pronounced velocity decoupling and shear dampening effects with intra-MB flow velocities over 200-fold lower than the main channel—allowing high channel flow rates for efficient exchange while preserving low-shear microenvironments, optimal for tissue culture. Additionally, tissues spatially compartmentalized in individual MBs are not dislodged under high flow conditions. This allowance for high channel flow rates, decoupled from the MB microenvironment, enables the use of millifluidic devices which are less difficult to manufacture and control than microfluidic devices. Simulations also showed that MBs with AR values between 2 and 3 offered a balance between nutrient transport and retention of cell-secreted factors. In contrast, rectilinear wells exhibited flow splitting and lactate accumulation at AR > 2, highlighting a key advantage of the spherical MB geometry. We fabricated a millifluidic MB device using fused deposition modeling (FDM) 3D printing and a novel molding strategy to create optically clear, leak-free flow channels. Murine salivary gland tissues cultured under flow in this device showed preserved acinar cell marker gene expression and reduced ductal markers, supporting the hypothesis that dynamic flow enhances tissue fidelity. This MB-fluidic platform enables scalable, high-content 3D culture systems suitable for organoid, tumor spheroid, and tissue mimetic applications in drug discovery and toxicology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Lab on a Chip
工程技术-化学综合
CiteScore
11.10
自引率
8.20%
发文量
434
审稿时长
2.6 months
期刊介绍:
Lab on a Chip is the premiere journal that publishes cutting-edge research in the field of miniaturization. By their very nature, microfluidic/nanofluidic/miniaturized systems are at the intersection of disciplines, spanning fundamental research to high-end application, which is reflected by the broad readership of the journal. Lab on a Chip publishes two types of papers on original research: full-length research papers and communications. Papers should demonstrate innovations, which can come from technical advancements or applications addressing pressing needs in globally important areas. The journal also publishes Comments, Reviews, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


