MAPL调节气真皮蛋白介导的mtDNA从溶酶体释放,以驱动热腐细胞死亡。
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
线粒体对细胞死亡的控制在从癌症到神经退行性疾病机制中起着至关重要的作用。线粒体锚定蛋白连接酶(MAPL)是一种线粒体外膜小泛素样修饰连接酶,是细胞存活的关键决定因素,但MAPL如何控制这一过程的命运尚不清楚。结合全基因组功能遗传筛选和细胞生物学方法,我们发现MAPL通过涉及线粒体和溶酶体的炎症途径诱导焦亡。MAPL过表达促进线粒体DNA在线粒体来源的囊泡中运输到溶酶体,溶酶体在一个需要气皮蛋白孔的过程中被渗透。这触发mtDNA释放到细胞质中,激活细胞死亡所需的DNA传感器cGAS。此外,多种帕金森病相关基因,包括VPS35和LRRK2,也调节mapl诱导的焦亡。值得注意的是,MAPL、LRRK2或VPS35的缺失抑制了原代巨噬细胞的炎症细胞死亡,将MAPL和线粒体-溶酶体途径置于免疫信号传导和细胞死亡的联系中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
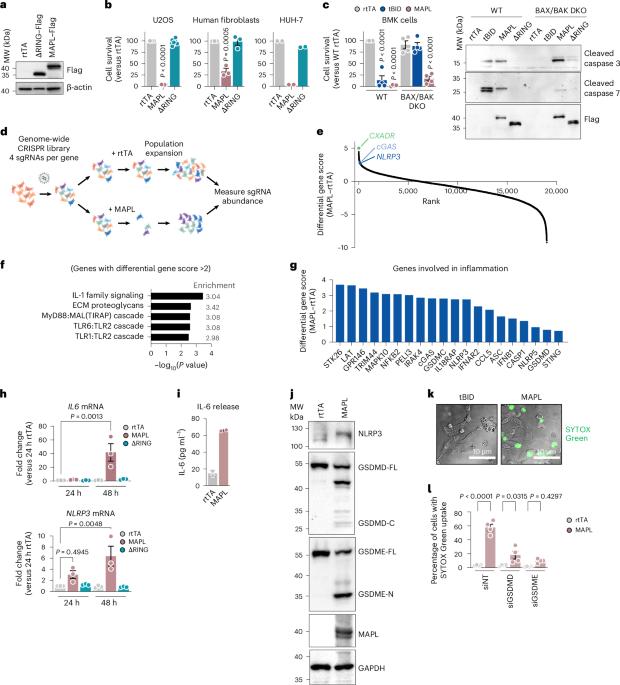
MAPL regulates gasdermin-mediated release of mtDNA from lysosomes to drive pyroptotic cell death
Mitochondrial control of cell death is of central importance to disease mechanisms from cancer to neurodegeneration. Mitochondrial anchored protein ligase (MAPL) is an outer mitochondrial membrane small ubiquitin-like modifier ligase that is a key determinant of cell survival, yet how MAPL controls the fate of this process remains unclear. Combining genome-wide functional genetic screening and cell biological approaches, we found that MAPL induces pyroptosis through an inflammatory pathway involving mitochondria and lysosomes. MAPL overexpression promotes mitochondrial DNA trafficking in mitochondrial-derived vesicles to lysosomes, which are permeabilized in a process requiring gasdermin pores. This triggers the release of mtDNA into the cytosol, activating the DNA sensor cGAS, required for cell death. Additionally, multiple Parkinson’s disease-related genes, including VPS35 and LRRK2, also regulate MAPL-induced pyroptosis. Notably, depletion of MAPL, LRRK2 or VPS35 inhibited inflammatory cell death in primary macrophages, placing MAPL and the mitochondria–lysosome pathway at the nexus of immune signalling and cell death. Nguyen, Collier et al. find a mitochondria–lysosome inflammatory pathway regulated by the SUMO E3 ligase MAPL, which promotes vesicular mtDNA transport to lysosomes and subsequent gasdermin-dependent lysosomal permeabilization to activate pyroptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


