钙网蛋白增强干恒温器应激肝癌细胞疫苗的治疗性免疫反应
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
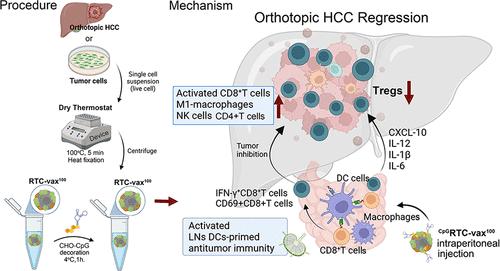
尽管全肿瘤细胞疫苗有望用于个体化癌症免疫治疗,但其有限的免疫原性和缺乏危险信号阻碍了其临床疗效。在此,我们介绍了一种基于时间效率(在2小时内)的干燥恒温器(DT)装置制备CpGRTC-vax100的方法,CpGRTC-vax100是一种治疗性疫苗,通过DT处理肿瘤细胞并进一步修饰胆固醇连接的cpg - odn,以消除原位肝细胞癌(HCC)。DT处理保留了肿瘤细胞形态的完整性和抗原活性,促进钙网蛋白向细胞表面的易位(约13倍),上调HSP70(约3.4倍),诱导DNA断裂,并在5分钟内立即灭活肿瘤细胞。这种方法通过增强免疫原性和暴露病原体相关和损伤相关的分子模式,克服了现有全细胞疫苗的局限性。这些变化导致肿瘤抗原有效摄取,树突状细胞活化,肿瘤中CXCL-10、Gzms-B、IL-6和IL-12水平升高。因此,t细胞在原位肝癌中的浸润增强。机制研究表明,NK细胞、CD4+T细胞和活化的CD8+T细胞被募集,同时肿瘤内Tregs减少。此外,CpGRTC-vax100激活患者来源的DC-T细胞,通过过继转移活化的DC-T细胞,在患者来源的异种移植模型中显示出有效的抗肿瘤作用。我们的研究结果强调了物理方法在增强肿瘤细胞疫苗免疫原性方面的潜力,以及它们在解决HCC治疗迫切需要方面的治疗前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Calreticulin Enhances Therapeutic Immune Responses of Dry Thermostat-Stressed Hepatocellular Carcinoma Cell Vaccine
Despite the promise of whole tumor cell vaccines in personalized cancer immunotherapy, their limited immunogenicity and lack of danger signals hinder their clinical efficacy. Herein, we introduce a time-efficient (within 2 h) dry thermostat (DT) device-based method to prepare CpGRTC-vax100, a therapeutic vaccine generated by treating tumor cells through DT and further decorated with cholesterol-linked CpG-ODNs, to eliminate orthotopic hepatocellular carcinoma (HCC). The DT treatment preserves tumor cell morphology integrity and antigenic activity, promotes the translocation of calreticulin to the cell surface (approximately 13-fold), upregulates HSP70 (around 3.4-fold), and induces DNA breaks while immediately inactivating tumor cells within 5 min. This approach overcomes the limitations of existing whole cell vaccines by enhancing immunogenicity and exposing pathogen-associated and damage-associated molecular patterns. These changes led to efficient tumor antigen uptake, dendritic cell activation, and increased levels of CXCL-10, Gzms-B, IL-6, and IL-12 in tumors. Consequently, the T-cell infiltration into orthotopic HCC is enhanced. Mechanistic studies reveal that NK cells, CD4+T cells, and activated CD8+T cells are recruited, alongside with reduced Tregs within the tumor. Furthermore, CpGRTC-vax100 activates patient-derived DC-T cells, demonstrating an efficient antitumor effect in patient-derived xenograft models through adoptive transfer of activated DC-T cells. Our findings highlight the potential of physical methods in enhancing tumor cell vaccine immunogenicity and their therapeutic promise for addressing the urgent needs of HCC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


