水环境中MXenes的缺陷驱动降解及其缓解策略:来自第一性原理的见解
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
MXenes由于其可调的表面化学,高导电性和易于溶液处理而引起了相当大的关注,使其成为广泛应用的有希望的候选者。MXenes在环境条件下的固有降解倾向限制了其成分的多样性,限制了其某些实际应用。我们的计算研究表明,无缺陷Ti3C2Tx的降解受到动力学限制,而普通缺陷显著降低了水侵蚀的激活障碍。通过从头算分子动力学模拟(AIMD)和热力学分析,我们发现钛空位VTi作为亚表面碳原子质子化的活性位点,削弱了与相邻Ti原子的键并加速了其释放。通过吸附的金属阳离子(如Li+, Na+, K+和Mg2+)对这些位点进行靶向钝化,预计可以通过抑制质子化和增加钛氧化屏障来有效减轻降解。这种稳定性源于两种协同效应:(i)由强偶极矩驱动的电子结构改变,这明显改变了功函数;(ii)限制水进入活性缺陷位点的位阻。我们还证明了碳空位VC显著地破坏了相邻Ti原子的稳定,降低了水攻击反应的能垒。用O或N等电负性物质取代VC并不能显著提高Ti3C2Tx的稳定性,这突出了碳亚晶格中任何缺陷的有害作用。由于VC通常是从前体相遗传而来,不能在后合成过程中被去除,因此在Mn+1AXn相合成过程中控制VC的浓度至关重要。我们的热力学分析表明,富a(例如富al)的合成条件大大增加了Mn+1AXn相大光谱中VC和VN缺陷的形成能量,为抑制缺陷和提高所得MXenes的耐久性提供了一种可推广的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
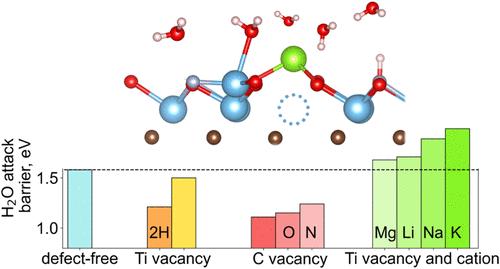
Defect-Driven Degradation of MXenes in Aqueous Environments and Mitigation Strategies: Insights from First-Principles
MXenes have attracted considerable attention due to their tunable surface chemistry, high electrical conductivity, and ease of solution processing, making them promising candidates for a wide array of applications. The inherent tendency of MXenes to degrade under environmental conditions constrains their compositional diversity and limits certain practical applications. Our computational study shows that degradation of defect-free Ti3C2Tx is kinetically limited, whereas common defects markedly lower the activation barriers for water attack. Using ab initio molecular dynamics simulations (AIMD) combined with thermodynamic analysis, we show that titanium vacancies VTi act as active sites for the protonation of subsurface carbon atoms, weakening the bonds with and accelerating the release of adjacent Ti atoms. Targeted passivation of these sites by adsorbed metal cations (e.g., Li+, Na+, K+, and Mg2+) is predicted to effectively mitigate degradation by suppressing protonation and increasing the barrier for Ti oxidation. This stabilization arises from two synergistic effects: (i) electronic structure modification driven by a strong dipole moment, which markedly shifts the work function, and (ii) steric hindrance that limits water access to reactive defect sites. We also demonstrate that carbon vacancies VC significantly destabilize adjacent Ti atoms, lowering the energy barrier for the water attack reaction. The substitution of VC with electronegative species such as O or N does not significantly improve the stability of Ti3C2Tx, highlighting the detrimental role of any defects in the carbon sublattice. Because VC are typically inherited from the precursor phase and cannot be removed during postsynthesis, controlling their concentration during Mn+1AXn phases synthesis is essential. Our thermodynamic analysis reveals that A-rich (e.g., Al-rich) synthesis conditions substantially increase the formation energy of VC and VN defects in a large spectrum of Mn+1AXn phases, providing a generalizable strategy for defect suppression and improved durability of the resulting MXenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


