纳米酶驱动Fenton-RAFT聚合对Caspase-3高灵敏度的PEC-CL双模生物传感
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
可逆加成-破碎链转移(RAFT)聚合为敏感生物传感中的信号放大提供了一种有效的策略。在此,我们开发了一种新型的光电化学-比色(PEC-CL)双模式生物传感平台,用于高灵敏度检测caspase-3活性,该平台基于纳米酶驱动的Fenton反应引发的RAFT聚合。具体来说,用链转移剂(CPAD)修饰磁性CuFe2O4纳米酶,形成CPAD-CuFe2O4,通过caspase-3可切割的DEVD肽链固定在传感界面上。引入caspase-3后,官能化CuFe2O4纳米酶被释放并催化Fenton反应生成羟基自由基(•OH),从而引发固定hemin的乙烯基单体(Hemin-VM)的RAFT聚合。将聚Hemin-VM (pHemin-VM)原位接枝到纳米酶表面,形成pHemin-VM/CuFe2O4复合材料,通过正反馈机制增强了•OH的生成,从而进一步放大了传感信号。最后,通过SnS2/MITO电极极性切换引起的PEC响应和3,3 ',5,5 ' -四甲基联苯胺(TMB)氧化产生的CL信号,灵敏地检测caspase-3。所提出的PEC - CL双模传感平台具有宽线性范围(PEC: 10-16 - 10-8 g mL-1, CL: 10-15-10-8 g mL-1)和低检出限(PEC为5.0 × 10-17 g mL-1, CL为2.7 × 10-16 g mL-1)的caspase-3活性测定。本研究提出了一种纳米酶驱动的Fenton化学启动RAFT聚合信号扩增策略,并扩展了其在生物传感领域的应用,为疾病生物标志物的双模式检测提供了一个强大的平台,推动了下一代生物传感器的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
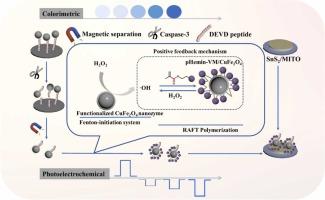
Nanozyme-driven fenton-RAFT polymerization for highly sensitive PEC–CL dual-mode biosensing of caspase-3
Reversible addition–fragmentation chain-transfer (RAFT) polymerization offers an effective strategy for signal amplification in sensitive biosensing. Herein, we developed a novel photoelectrochemical–colorimetric (PEC–CL) dual-mode biosensing platform for the highly sensitive detection of caspase-3 activity, based on the RAFT polymerization initiated by nanozyme-driven Fenton reaction. Specifically, a magnetic CuFe2O4 nanozyme was modified with chain transfer agents (CPAD) to form CPAD-CuFe2O4, which was immobilized on the sensing interface via a caspase-3-cleavable DEVD peptide linkage. Upon the introduction of caspase-3, the functionalized CuFe2O4 nanozyme was released and catalyzed the Fenton reaction to generate hydroxyl radicals (•OH), thereby initiating RAFT polymerization of Hemin-fixed vinyl monomers (Hemin-VM). The resulting poly(Hemin-VM) (pHemin-VM) was in situ grafted onto the surface of nanozyme, forming a pHemin-VM/CuFe2O4 composite that enhanced •OH generation through a positive feedback mechanism, consequently further amplifying the sensing signals. Finally, caspase-3 was sensitively detected through PEC responses arising from polarity switching at the SnS2/MITO electrode and CL signals generated by the oxidation of 3,3’,5,5’-tetramethylbenzidine (TMB). The proposed PEC–CL dual-mode sensing platform exhibited a wide linear range (PEC: 10−16–10−8 g mL−1, CL: 10−15–10−8 g mL−1) and low detection limit (5.0 × 10−17 g mL−1 for PEC, and 2.7 × 10−16 g mL−1 for CL) for caspase-3 activity assay. This study introduced a nanozyme-driven Fenton chemistry-initiated RAFT polymerization signal amplification strategy, and expanded its application in biosensing, offering a robust platform for dual-mode detection of disease biomarkers and advancing next-generation biosensor development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


