中熵氟化氧衍生的催化纳米颗粒增强氢氧化镁脱氢动力学
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
氢化镁的储氢容量为7.6 wt%,因此被认为是固态储氢的潜在候选材料。然而,脱氢动力学缓慢严重阻碍了其实际应用。本文合成了一种中熵氟化氧(Ti0.25V0.25Nb0.25Zr0.25)O1.5F1.5 (MEOF)作为加速MgH2氢释放的添加剂。将制备的氟化氧(10 wt%)加入MgH2后,脱氢动力学优异,活化能为67.7 kJ mol−1。即使经过50次脱氢/加氢循环,脱氢活化能和容量保留率仍分别保持在70.7 kJ mol−1和98.5 %;具有优异的循环耐久性。进一步研究表明,在制备meof掺杂MgH2复合材料的过程中,原位形成的极度分散的过渡金属相协同催化了显著的动力学增强。同时,循环耐久性的提高是由于原位生成的MgF2和MgO对晶体生长和粉末团聚的抑制作用。这些结果揭示了多相协同对提高氢氧化镁脱氢性能的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
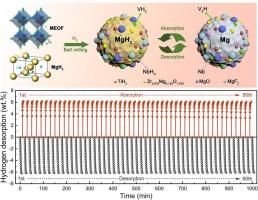
Medium-entropy oxyfluoride-derived catalytic nanoparticles for enhancement of dehydrogenation kinetics of magnesium hydride
Magnesium hydride has a hydrogen capacity of 7.6 wt%, thereby being believed as a potential candidate for solid-state hydrogen storage. Nevertheless, the slow dehydrogenation kinetics severely hampers its practical utilizations. Herein, a medium-entropy oxyfluoride (Ti0.25V0.25Nb0.25Zr0.25)O1.5F1.5 (MEOF) is synthesized as an additive to accelerate hydrogen release from MgH2. After introducing the as-prepared oxyfluoride (10 wt%) into MgH2, the superior dehydrogenation kinetics with an activation energy of 67.7 kJ mol−1 is achieved. Even after fifty de/hydrogenation cycles, the dehydrogenation activation energy and capacity retention still remain at 70.7 kJ mol−1 and 98.5 %, respectively; showing excellent cycle durability. Further investigation demonstrates that the significant kinetic enhancement is attributed to the synergistic catalysis of extremely dispersed transition metal phases which are in-situ formed in the preparation process of MEOF-doped MgH2 composite. Meanwhile, the improvement of cycling durability is owed to the inhibitory effect of in-situ generated MgF2 and MgO on crystallite growth and powder agglomeration. These results bring insight into the importance of multiphase synergy in improving hydrogen desorption performance of magnesium hydride.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


