用IrO2-Ta2O5|Ti阳极和碳纸阴极联合去除阿莫西林和酮罗拉酸
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
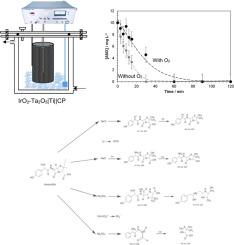
众所周知,水资源中的污染物会造成严重的环境和健康问题。尽管使用了不同的方法来消除这些化学物质,但新出现的污染物通常是持久的,特别是在传统的水处理技术中。电氧化(EO)是一种简单的技术,已被证明对水中的药物降解有效。本研究采用IrO2-Ta2O5|Ti阳极(含18.79% Ti, 58.09% O, 7.06% Ir和2.36% Ta),粗糙度为252.6 μm,表面积为0.0667 cm2,粗糙度系数为0.07575,在不同的设置下,通过将其与钛网或碳纸(CP)作为阴极,在恒定气流下或不恒定气流下,研究了EO的性能。通过对过氧化氢(H2O2)、羟基自由基(•OH)和活性氯的积累进行监测,以评估每种装置在两种不同介质(0.1 M NaCl和0.1 M Na2SO4)中的性能。采用超高效液相色谱-紫外-可见光谱法(UPLC-UV/Vis)测定了样品中阿莫西林(AMX)和酮酸(KET)的去除率。在IrO2-Ta2O5|Ti||CP体系中,10 mg L-1 AMX在0.1 M NaCl和0.1 M Na2SO4中分别作用6 min和30 min,去除率均为100%。相比之下,10 mg L-1 KET在0.1 M NaCl中5 min的去除率为100%,在0.1 M Na2SO4中120 min的去除率为82.5%,在不到10 min的时间内毒性相应降低。利用总有机碳(TOC)分析确定AMX和KET溶液的矿化程度。结果表明,在0.1 M的Na2SO4介质中,H2O2和•OH自由基的生成率较高。此外,在曝气系统中,CP处的2e-氧还原反应(ORR)有助于药物的更快降解。氯的产生加速了两种药物在NaCl介质中的消失。最后,结合生成的界面氧化剂(H2O2、•OH、Cl2/HClO)以及通过高效液相色谱-质谱联用(HPLC-MS/MS)鉴定的相应副产物,提出了AMX在Na2SO4中降解和KET在NaCl中降解的反应路线。在不到10分钟的时间内观察到毒性下降。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Removal of amoxicillin and ketorolac combining an IrO2-Ta2O5|Ti anode with a carbon paper cathode
Contaminants in water resources are known to cause serious environmental and health issues. Despite using different methods to eliminate such chemicals, emerging contaminants are usually persistent, especially in conventional water treatment technologies. Electro-oxidation (EO) is a straightforward technique that has been proven effective for degrading pharmaceuticals in water. In this work, the performance of EO has been investigated using an IrO2-Ta2O5|Ti anode containing 18.79 % Ti, 58.09 % O, 7.06 % Ir, and 2.36 % Ta, with a roughness of 252.6 μm, a surface area of 0.0667 cm2, and a roughness factor of 0.07575, in different setups by combining it with either a titanium mesh or carbon paper (CP) as a cathode, with or without a constant air flow rate. The accumulation of hydrogen peroxide (H2O2), hydroxyl radical (•OH), and active chlorine was monitored to evaluate the performance of each setup in two different media (0.1 M NaCl and 0.1 M Na2SO4). The EO process was conducted at a cell potential of 2.5 V for 120 min, and the removal of amoxicillin (AMX) and ketorolac (KET) was assessed by Ultraperformance Liquid Chromatography coupled to UV–Vis Spectrometry (UPLC-UV/Vis). In this sense, using the IrO2-Ta2O5|Ti||CP system, 10 mg l-1 AMX showed a removal efficiency of 100 % after 6 min in 0.1 M NaCl and after 30 min in 0.1 M Na2SO4. In comparison, 10 mg l-1 KET showed a removal efficiency of 100 % after 5 min in 0.1 M NaCl and 82.5 % after 120 min in 0.1 M Na2SO4, with a corresponding decrease in toxicity within <10 min. The mineralization of AMX and KET solutions was determined using total organic carbon (TOC) analysis. Our results showed that higher H2O2 and •OH radicals were produced in a 0.1 M Na2SO4 medium. Moreover, in aerated systems, the 2e- oxygen reduction reaction (ORR) at CP contributed to the faster degradation of the drugs. The chlorine production accelerated the disappearance of both drugs in the NaCl medium. Finally, reaction routes for the AMX degradation in Na2SO4 and KET degradation in NaCl are proposed, considering the interfacial oxidants generated (H2O2, •OH, Cl2/HClO) and the corresponding by-products identified by High Performance Liquid Chromatography coupled to tandem Mass Spectrometry (HPLC-MS/MS). The decrease in toxicity was observed within <10 min.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


