BDD阳极的结构-功能关系:电化学生成去除磺胺甲恶唑氧化剂的见解
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
电化学高级氧化过程(EAOPs)的效率很大程度上取决于阳极-电解质界面上活性物质的产生。在这项研究中,我们系统地评估了五种不同碳杂化比(sp³/sp²)、硼掺杂水平(500-10,000 ppm)和衬底材料(二氧化硅或铌)的商业硼掺杂金刚石(BDD)阳极,以研究它们产生氧化剂和降解抗生素磺胺甲恶唑(SMX)的能力。羟基自由基(OH)、过硫酸盐(S₂O₈²⁻)和次氯酸盐(ClO⁻)的产生分别用EPR光谱法、UV-Vis分光光度法和电位滴定法进行了定量分析。结果表明,阳极的组成对氧化剂的生成有显著影响。阳极浓度为500ppm B, sp³/sp²比高,衬底为二氧化硅,由于良好的表面性能和高的电荷转移电阻,产生了最高的●OH和过硫酸盐。相反,含有2500ppm B的阳极产生了最高的氯-毒血症浓度,大大增强了含氯电解质中SMX的降解。形态学和润湿性分析表明,硼掺杂的增加促进了棒状表面结构和更强的疏水性,促进了活性氯(ACS)的脱附。SMX的去除效率取决于形成的主要氧化剂:活性氧(ROS)在硫酸盐介质中占主导地位,而ACS在氯化物介质中更有效。这些发现证明了BDD阳极的结构和电化学性能如何控制选择性氧化剂的形成和污染物的降解。该研究为BDD电极的结构-功能关系提供了新的见解,并强调了电极设计在优化EAOPs的性能和选择性方面的重要性,以实现可持续水处理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
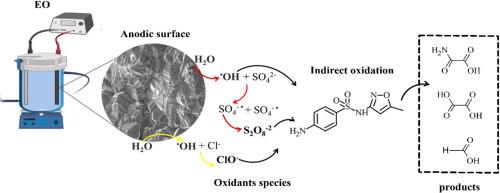
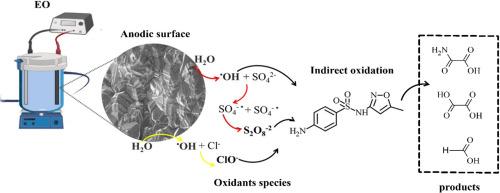
Structure-function relationships in BDD anodes: Insights into electrochemical generation of oxidants for sulfamethoxazole removal
The efficiency of electrochemical advanced oxidation processes (EAOPs) strongly depends on the electrogeneration of reactive species at the anode–electrolyte interface. In this study, we systematically evaluated five commercial boron-doped diamond (BDD) anodes with varying carbon hybridization ratios (sp³/sp²), boron doping levels (500–10,000 ppm), and substrate materials (silica or niobium) to investigate their ability to generate oxidants and degrade the antibiotic sulfamethoxazole (SMX). The production of hydroxyl radicals (∙OH), persulfate (S₂O₈²⁻), and hypochlorite (ClO⁻) was quantified using EPR spectroscopy, UV–Vis spectrophotometry, and potentiometric titration, respectively. The results demonstrated that the composition of the anode significantly influences oxidant generation. The anode with 500 ppm B, a high sp³/sp² ratio, and a silica substrate exhibited the highest ∙OH and persulfate production, attributed to favorable surface properties and high charge transfer resistance. In contrast, the anode with 2500 ppm B produced the highest ClO⁻ concentrations, substantially enhancing SMX degradation in chloride-containing electrolytes. Morphological and wettability analyses revealed that increased boron doping promoted rod-like surface structures and greater hydrophobicity, facilitating the desorption of active chlorine species (ACS). SMX removal efficiency varied depending on the predominant oxidants formed: reactive oxygen species (ROS) dominated in sulfate media, whereas ACS were more effective in chloride media. These findings demonstrate how the structural and electrochemical properties of BDD anodes govern selective oxidant formation and pollutant degradation. This study provides new insights into structure–function relationships in BDD electrodes and underscores the importance of electrode design in optimizing the performance and selectivity of EAOPs for sustainable water treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


