烧碱氧化镁的氨基酸水化:高效转化和净化的双重功能
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
烧氧化镁(MgO)水化制氢氧化镁(Mg(OH)2)是镁化学工程的基础,但由于未反应的MgO形成致密的产物层和天然菱镁矿中持续存在的杂质挑战,它面临动力学限制。本研究提出了一种氨基酸介导MgO水化和原位纯化同步增强的方法。对非极性、极性中性、碱性和酸性8种氨基酸的筛选表明,非极性氨基酸(如甘氨酸)显著促进水合作用和杂质分离,而极性碱性氨基酸由于其强表面吸附而阻碍水合作用。机理表征表明,氨基酸通过羧基介导的静电吸附和与MgO表面的氢键作用调节Mg2+的溶解-沉淀途径,促进Mg(OH)2的非均相成核,从而有效分离高密度杂质。通过响应面法优化,该工艺水化效率为99.12%,Mg(OH)2纯度为99.03%,杂质含量低。中试试验表明,每吨氧化镁的利润为395美元,为低品位菱镁矿的高价值利用开辟了一条可行的技术路线。这项工作揭示了氨基酸在水合动力学和杂质分离中的双重作用,为高效高纯度Mg(OH)2的生产提供了一种创新的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
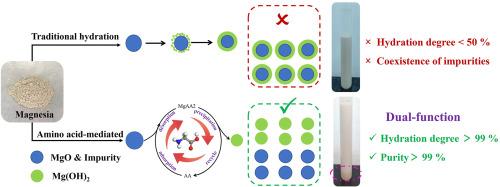
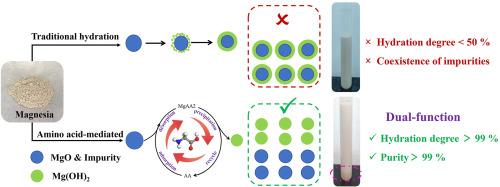
Amino acid mediated hydration of caustic calcined magnesia: dual-function for efficient conversion and purification
Caustic calcined magnesia (MgO) hydration to magnesium hydroxide (Mg(OH)2) is fundamental in magnesium chemical engineering, yet it faces kinetic limitations from the dense product layer forming on unreacted MgO and persistent impurity challenges from natural magnesite. This study has presented an approach for synchronous enhancement of MgO hydration and in-situ purification via amino acid mediation. Screening eight amino acids across non-polar, polar neutral, basic, and acidic categories revealed that non-polar amino acids (e.g., glycine) notably promoted hydration and impurity separation, whereas polar basic amino acids hinder hydration due to strong surface adsorption. Mechanistic characterization showed amino acids regulated the Mg2+ dissolution-precipitation pathway through carboxyl-group-mediated electrostatic adsorption and hydrogen bonding with the MgO surface, facilitating heterogeneous nucleation of Mg(OH)2 for effective separation from high-density impurities. Optimized by response surface methodology, the process achieved 99.12 % hydration efficiency and 99.03 % pure Mg(OH)2 with low impurity levels. Pilot-scale experiments demonstrated a profit of 395 US dollars per ton of MgO, establishing a viable technical route for high-value utilization of low-grade magnesite. This work uncovered the dual role of amino acids in mediating both hydration kinetics and impurity segregation, offering an innovative solution for efficient high-purity Mg(OH)2 production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


