hesc来源的多巴胺祖细胞治疗帕金森病的1/2a期临床试验
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
长期以来,帕金森病(PD)一直被认为是细胞替代疗法的合适候选者。我们从人胚胎干细胞中获得了高纯度的多巴胺能祖细胞(A9-DPCs),并在一项针对PD患者的单中心、开放标签、剂量递增的1/2a期试验(NCT05887466)中评估了它们的安全性和实验性有效性。12例中重度PD患者接受低剂量(315万个细胞,n = 6)或高剂量(630万个细胞,n = 6)免疫抑制的A9-DPC双侧壳核移植。未观察到剂量限制性毒性或移植物相关不良事件。12个月时,停药期运动障碍学会统一帕金森病评定量表(MDS-UPDRS)第三部分评分和Hoehn和Yahr分期均有所改善,高剂量组运动改善更大。多巴胺转运体正电子发射断层扫描(PET)成像显示,移植后高剂量组后壳核摄取增加,摄取更大,支持移植物存活。这些结果表明,双侧A9-DPC移植是安全的,并可能改善PD患者的帕金森运动症状。本文章由计算机程序翻译,如有差异,请以英文原文为准。
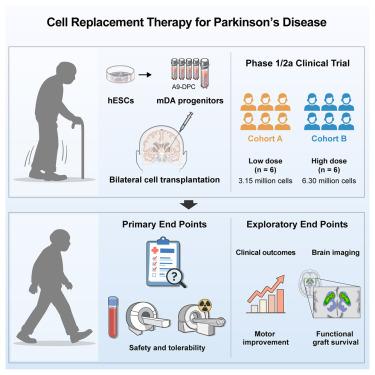
Phase 1/2a clinical trial of hESC-derived dopamine progenitors in Parkinson’s disease
Parkinson’s disease (PD) has long been considered an appropriate candidate for cell replacement therapy. We generated high-purity dopaminergic progenitors (A9-DPCs) from human embryonic stem cells and evaluated their safety and exploratory efficacy in a single-center, open-label, dose-escalation phase 1/2a trial (NCT05887466) for PD patients. Twelve patients with moderate-to-severe PD received bilateral putamen transplantation of low-dose (3.15 million cells; n = 6) or high-dose (6.30 million cells; n = 6) A9-DPC with immunosuppression. No dose-limiting toxicities or graft-related adverse events were observed. At 12 months, off-medication Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III scores and Hoehn and Yahr stage improved, with greater motor improvements in the high-dose group. Dopamine transporter positron emission tomography (PET) imaging showed increased posterior putamen uptake with greater uptake in the high-dose group after transplantation, supporting graft survival. These findings indicate that bilateral transplantation of A9-DPC is safe and may improve parkinsonian motor symptoms in patients with PD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


