碳掺杂氮化碳的电子自旋极化增强PMS活化对新出现污染物的深度降解
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
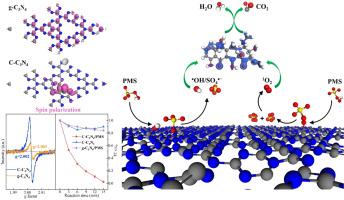
过渡金属基催化剂在过氧单硫酸盐(PMS)活化中的稳定性差和金属浸出给新兴污染物(ECs)的降解带来了实际挑战。探索具有高活性和稳定性的非金属催化剂是十分必要的。本文采用样品热聚合法制备了掺杂碳的石墨化氮化碳(C-C3N4)。通过多种表征结合密度泛函理论(DFT)计算,详细分析了C-C3N4的形貌和化学结构。在C-C3N4/PMS体系中,四环素(TC)在15 min内几乎100%脱除,50 min内矿化率高达70%。实验表明,C-C3N4的连续反应和循环使用表现出优异的稳定性,360 min后四环素(TC)的去除率仍超过80%。测试了C-C3N4对不同水源、阴离子和污染物的高适用性。定性和定量分析活性氧(ROSs)证实了•OH, SO4•−,1O2的生成增强。C掺杂导致电子自旋极化,调节电子结构,从而增强PMS与C- c3n4之间的电子转移。原位分析结合DFT计算发现,掺杂C是PMS活化的活性位点,PMS在掺杂C和N - (C)2上的双位点吸附大大降低了生成•OH的能垒(从1.52 eV降至0.27 eV)。通过计算Fukui函数,结合LC-MS检测中间体,得出了详细的降解途径。该研究为开发高效的无金属催化剂以及通过类芬顿催化深度降解ECs提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electron spin polarization of C-doped carbon nitride enhancing PMS activation for deep degradation of Emerging Contaminants
The poor stability and metal leaching of transition metal-based catalysts for peroxymonosulfate (PMS) activation cause practical challenges in Emerging Contaminants (ECs) degradation. It is necessary to explore non-metallic catalyst with high activity and stability. Herein, carbon-doped graphitic carbon nitride (C-C3N4) is synthesized through sample thermal polymerization. The morphology and chemical structure of C-C3N4 are analyzed in detail through multiple characterizations combining density functional theory (DFT) calculation. The tetracycline (TC) can be nearly 100% removed in 15 min with the high mineralization rate (∼70%) in 50 min by C-C3N4/PMS system. The continuous reaction and recycle using experiments exhibit the excellent stability of C-C3N4 and the tetracycline (TC) remove rate still exceeds 80% after 360 min. The high applicability of C-C3N4 to different water sources, anions and pollutants is also tested. Qualitative and quantitative analysis of reactive oxygen species (ROSs) proves the enhanced generation of •OH, SO4•−, 1O2. C doping leads to the electron spin polarization and regulates electronic structure thus enhances the electron transfer between PMS and C-C3N4. In situ analysis combining DFT calculation identifies that doped C is the active site for PMS activation and dual-site adsorption of PMS on doped C and N‒(C)2 greatly decreases the energy barrier for •OH generation (from 1.52 to 0.27 eV). Fukui function calculation combining the intermediate detection by LC-MS concludes the detailed degradation pathway. This study provides a valuable insight for the development of efficient metal-free catalysts and the deep degradation of ECs through Fenton-like catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


