固体电解质在抑制可扩展H2O2电合成焦耳热效应中的作用
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
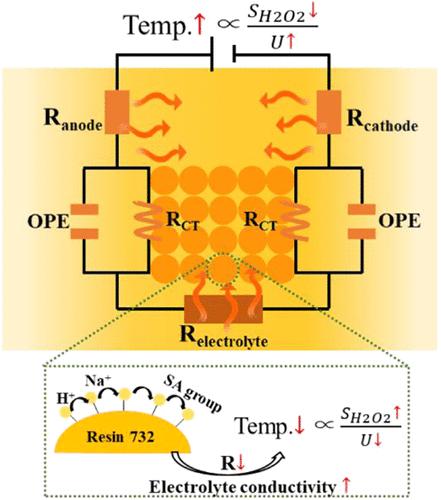
通过氧还原电合成过氧化氢(H2O2)有可能彻底改变传统的化学合成。然而,这一目标经常受到大规模生产过程中能源效率下降的阻碍,而能源效率是衡量H2O2电合成经济可行性的指导性指标。该研究确定焦耳加热是导致效率损失的主要原因,因为焦耳加热加速了H2O2的分解,并导致了大量的电压下降。热学和阻抗分析表明,通过提高电解质电导率和优化反应器结构来降低内阻,可以显著抑制焦耳加热。其中,结构优化是降低内阻的瓶颈,而提高电解质电导率是进一步提高的关键。为了平衡电合成性能和环境可持续性,提出了一种na2so4 -强酸性阳离子交换树脂(SAC)复合固体电解质,大大减少了盐基液体电解质的使用。这种方法可以在工业电流密度下高效稳定地生产H2O2,为其可扩展应用提供了可行的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Role of Solid Electrolytes in Suppressing Joule Heating Effect for Scalable H2O2 Electrosynthesis
The electrosynthesis of hydrogen peroxide (H2O2) via oxygen reduction has the potential to revolutionize traditional chemical synthesis. However, this objective is often hindered by the decline in energy efficiency during large-scale production, a guiding metric for the economic feasibility of H2O2 electrosynthesis. This study identifies Joule heating as the primary contributor to efficiency loss as it accelerates H2O2 decomposition and induces substantial voltage drops. Thermal and impedance analyses reveal that Joule heating can be significantly suppressed by enhancing electrolyte conductivity and optimizing reactor structure to reduce internal resistance. Among these strategies, structural optimization shows a bottleneck in reducing internal resistance, while electrolyte conductivity enhancement is the key to further improvement. To balance electrosynthesis performance with environmental sustainability, a Na2SO4-strongly acidic cation exchange resin (SAC) composite solid electrolyte is proposed, significantly reducing the use of salt-based liquid electrolytes. This approach enables efficient and stable H2O2 production at an industrial current density, providing a feasible pathway for its scalable application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


