以聚乙烯为原料合成聚酯的混合二羧酸
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
为了摆脱目前以化石为基础的线性塑料价值链,我们的目标是通过聚乙烯(PE)废物的氧化转化生产二羧酸单体,如琥珀酸和己二酸。然而,这种技术的一个缺点是,与基于化石的类似物相比,它产生了不同链长的二羧酸的混合物。因此,我们的目标是探索混合二羧酸直接应用于聚酯合成的潜力。以1,4-丁二醇为共聚单体,由不同链长(C4-C10)和链长分布(1,3,5和7二酸)的二羧酸合成一系列脂肪族聚酯,比较了这些聚合物的物理性质。此外,还从聚乙烯(PE)的氧化转化得到的二羧酸混合物中合成了聚酯。采用差示扫描量热法(DSC)、凝胶渗透色谱法(GPC)、x射线衍射法(XRD)、红外光谱法(IR)、核磁共振(NMR)和热重分析(TGA)对聚合物进行了表征。使用混合二羧酸原料提高了生物降解性,但降低了聚合物的熔化温度。这可以通过使用更坚硬的二醇来补偿,例如对苯二甲酸二氢乙酯(bet)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
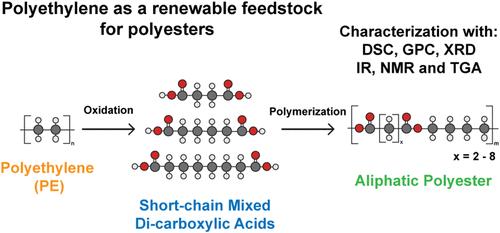
Mixed Dicarboxylic Acids Derived from Polyethylene as a Feedstock for the Synthesis of Polyesters
To move away from the currently linear fossil-based plastic value chain, we aim to produce dicarboxylic acid monomers, such as succinic and adipic acid, by the oxidative conversion of polyethylene (PE) wastes. However, a drawback of this technology is that a mixture of dicarboxylic acids of various chain lengths is produced, in contrast to their fossil-based analogs. Therefore, we aim to explore the potential of applying mixed dicarboxylic acids directly in polyester synthesis. The physical properties of these polymers were compared by synthesizing a range of aliphatic polyesters from dicarboxylic acids with a variation in chain length (i.e., C4–C10) and chain length distributions (i.e., 1, 3, 5, and 7 diacids) with 1,4-butanediol as the comonomer. In addition, a polyester was synthesized from a mix of dicarboxylic acids derived from the oxidative conversion of polyethylene (PE). The polymers were characterized with differential scanning calorimetry (DSC), gel permeation chromatography (GPC), X-ray diffraction (XRD), infrared (IR) spectroscopy, nuclear magnetic resonance (NMR), and thermogravimetric analysis (TGA). Using a mixed dicarboxylic acid feedstock enhances the biodegradability but lowers the melting temperature of the polymers made. This can be compensated by the use of a more rigid diol, such as bis-hydroxyethyl terephthalate (BHET).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


